The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
The Staphylococcus aureus secretome - TI Pharma
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 2<br />
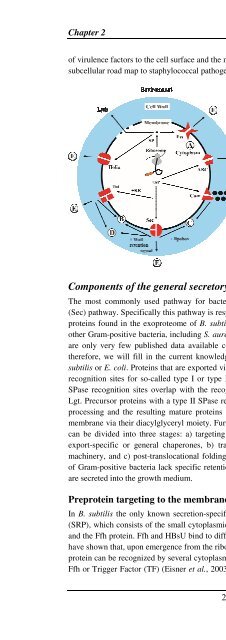
of virulence factors to the cell surface and the milieu of the host, Figure 4 can be regarded as a<br />
subcellular road map to staphylococcal pathogenesis.<br />
Components of the general secretory (Sec) Pathway<br />
26<br />
Figure 4. <strong>The</strong> staphylococcal “pathways to<br />
pathogenesis”. Schematic representation of a<br />
staphylococcal cell with potential pathways for<br />
protein sorting and secretion. (A) Proteins<br />
without signal peptide reside in the cytoplasm.<br />
(B) Proteins with one or more transmembrane<br />
spanning domains can be inserted into the<br />
membrane via the Sec, Tat or Com pathways. (C)<br />
Lipoproteins are exported via the Sec pathway<br />
and after lipid-modification anchored to the<br />
membrane. (D) Proteins with cell wall retention<br />
signals are exported via the Sec, Tat or Com<br />
pathways and retained in the cell wall via<br />
covalent-, or high-affinity binding to cell wall<br />
components. (E) Exported proteins with a signal<br />
peptide and without a membrane or cell wall<br />
retention signal can be secreted into the<br />
extracellular milieu via the various indicated<br />
pathways.<br />
<strong>The</strong> most commonly used pathway for bacterial protein transport is the general secretory<br />
(Sec) pathway. Specifically this pathway is responsible for the secretion of the majority of the<br />
proteins found in the exoproteome of B. subtilis and this is probably also the case for most<br />
other Gram-positive bacteria, including S. <strong>aureus</strong> (Tjalsma et al., 2004). Unfortunately, there<br />
are only very few published data available concerning the Sec pathway of S. <strong>aureus</strong> and,<br />
therefore, we will fill in the current knowledge gaps with data obtained from studies in B.<br />
subtilis or E. coli. Proteins that are exported via the Sec-pathway contain signal peptides with<br />
recognition sites for so-called type I or type II signal peptidases (SPases). Notably, type II<br />
SPase recognition sites overlap with the recognition sites for the diacylglyceryl transferase<br />
Lgt. Precursor proteins with a type II SPase recognition sequence are lipid-modified prior to<br />
processing and the resulting mature proteins are retained as lipopoteins in the cytoplasmic<br />
membrane via their diacylglyceryl moiety. Furthermore, the Sec-dependent export of proteins<br />
can be divided into three stages: a) targeting to the membrane translocation machinery by<br />
export-specific or general chaperones, b) translocation across the membrane by the Sec<br />
machinery, and c) post-translocational folding and modification. If the translocated proteins<br />
of Gram-positive bacteria lack specific retention signals for the membrane or cell wall, they<br />
are secreted into the growth medium.<br />
Preprotein targeting to the membrane<br />
In B. subtilis the only known secretion-specific chaperone is the signal recognition particle<br />
(SRP), which consists of the small cytoplasmic RNA (scRNA), the histon-like protein HBsU<br />
and the Ffh protein. Ffh and HBsU bind to different moieties of the scRNA. Studies in E. coli<br />
have shown that, upon emergence from the ribosome, the signal peptide of a nascent secretory<br />
protein can be recognized by several cytoplasmic chaperones and/or targeting factors, such as<br />
Ffh or Trigger Factor (TF) (Eisner et al., 2003). In contrast to Ffh, which is required for co-