142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Poly(macromonomers), Homo- and Copolymerization 155<br />
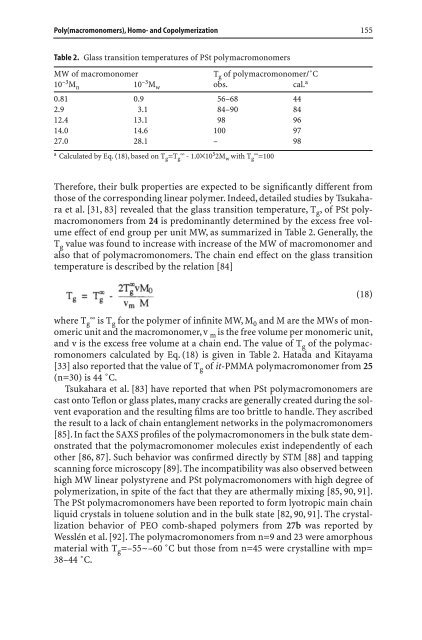
Table 2. Glass transition temperatures of PSt polymacromonomers<br />
MW of macromonomer<br />
10<br />
Tg of polymacromonomer/˚C<br />
–3Mn 10 –3Mw obs. cal. a<br />
0.81 0.9 56–68 44<br />
2.9 3.1 84–90 84<br />
12.4 13.1 98 96<br />
14.0 14.6 100 97<br />
27.0 28.1 – 98<br />
a Calculated by Eq. (18), based on Tg =T g ¥ - 1.0´10 5 2Mw with T g ¥ =100<br />
Therefore, their bulk properties are expected to be significantly different from<br />
those of the correspond<strong>in</strong>g l<strong>in</strong>ear polymer. Indeed, detailed studies by Tsukahara<br />
et al. [31, 83] revealed that the glass transition temperature, T g , of PSt polymacromonomers<br />
from 24 is predom<strong>in</strong>antly determ<strong>in</strong>ed by the excess free volume<br />
effect of end group per unit MW, as summarized <strong>in</strong> Table 2. Generally, the<br />
T g value was found to <strong>in</strong>crease with <strong>in</strong>crease of the MW of macromonomer and<br />
also that of polymacromonomers. The cha<strong>in</strong> end effect on the glass transition<br />
temperature is described by the relation [84]<br />
(18)<br />
where T g ¥ is Tg for the polymer of <strong>in</strong>f<strong>in</strong>ite MW, M 0 and M are the MWs of monomeric<br />
unit and the macromonomer, v m is the free volume per monomeric unit,<br />
and v is the excess free volume at a cha<strong>in</strong> end. The value of T g of the polymacromonomers<br />
calculated by Eq. (18) is given <strong>in</strong> Table 2. Hatada and Kitayama<br />
[33] also reported that the value of T g of it-PMMA polymacromonomer from 25<br />
(n=30) is 44 ˚C.<br />
Tsukahara et al. [83] have reported that when PSt polymacromonomers are<br />
cast onto Teflon or glass plates, many cracks are generally created dur<strong>in</strong>g the solvent<br />
evaporation and the result<strong>in</strong>g films are too brittle to handle. They ascribed<br />
the result to a lack of cha<strong>in</strong> entanglement networks <strong>in</strong> the polymacromonomers<br />
[85]. In fact the SAXS profiles of the polymacromonomers <strong>in</strong> the bulk state demonstrated<br />
that the polymacromonomer molecules exist <strong>in</strong>dependently of each<br />
other [86, 87]. Such behavior was confirmed directly by STM [88] and tapp<strong>in</strong>g<br />
scann<strong>in</strong>g force microscopy [89]. The <strong>in</strong>compatibility was also observed between<br />
high MW l<strong>in</strong>ear polystyrene and PSt polymacromonomers with high degree of<br />
polymerization, <strong>in</strong> spite of the fact that they are athermally mix<strong>in</strong>g [85, 90, 91].<br />
The PSt polymacromonomers have been reported to form lyotropic ma<strong>in</strong> cha<strong>in</strong><br />
liquid crystals <strong>in</strong> toluene solution and <strong>in</strong> the bulk state [82, 90, 91]. The crystallization<br />
behavior of PEO comb-shaped polymers from 27b was reported by<br />
Wesslén et al. [92]. The polymacromonomers from n=9 and 23 were amorphous<br />
material with T g=–55~–60 ˚C but those from n=45 were crystall<strong>in</strong>e with mp=<br />
38–44 ˚C.