142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
34 B. Charleux, R. Faust<br />
for the coupled products with acetoxy and methacryloyloxy functionality<br />
(yield>95%). For the coupled product with the styryl term<strong>in</strong>al group, the yield<br />
was lower (~90%). Structural analysis us<strong>in</strong>g 1 H NMR spectroscopy was performed<br />
after separation of the ma<strong>in</strong> product by preparative gel permeation<br />
chromatography. F n was close to the theoretical value 4, <strong>in</strong>dicat<strong>in</strong>g that the f<strong>in</strong>al<br />
product had the expected four arm structure with one functional group at the<br />
end of each arm.<br />
2.3.1.2<br />
Poly(isobutylene) n<br />
In view of the excellent shear stability of silicone oils, it was theorized that shear<br />
stable multiarm star PIBs could be prepared us<strong>in</strong>g cyclosiloxane cores [50]. The<br />
synthesis was accomplished <strong>in</strong> two steps. First, allyl term<strong>in</strong>ated PIB of desired<br />
MW was prepared by react<strong>in</strong>g liv<strong>in</strong>g PIB with trimethylallylsilane. L<strong>in</strong>k<strong>in</strong>g was<br />
effected by hydrosilylation of the allyl-functional PIB with cyclosiloxanes carry<strong>in</strong>g<br />
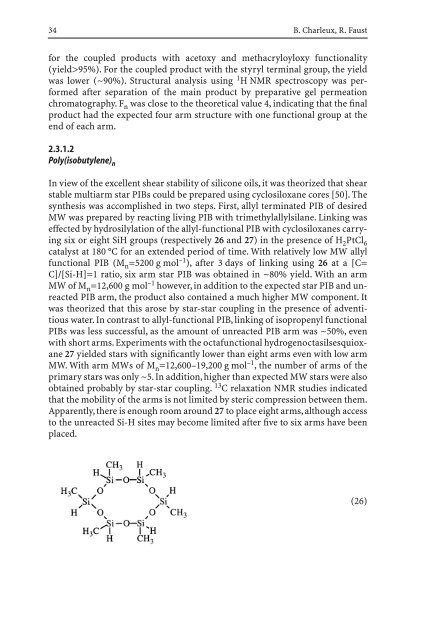
six or eight SiH groups (respectively 26 and 27) <strong>in</strong> the presence of H 2PtCl 6<br />
catalyst at 180 °C for an extended period of time. With relatively low MW allyl<br />
functional PIB (M n=5200 g mol –1 ), after 3 days of l<strong>in</strong>k<strong>in</strong>g us<strong>in</strong>g 26 at a [C=<br />
C]/[Si-H]=1 ratio, six arm star PIB was obta<strong>in</strong>ed <strong>in</strong> ~80% yield. With an arm<br />
MW of M n=12,600 g mol –1 however, <strong>in</strong> addition to the expected star PIB and unreacted<br />
PIB arm, the product also conta<strong>in</strong>ed a much higher MW component. It<br />
was theorized that this arose by star-star coupl<strong>in</strong>g <strong>in</strong> the presence of adventitious<br />
water. In contrast to allyl-functional PIB, l<strong>in</strong>k<strong>in</strong>g of isopropenyl functional<br />
PIBs was less successful, as the amount of unreacted PIB arm was ~50%, even<br />
with short arms. Experiments with the octafunctional hydrogenoctasilsesquioxane<br />
27 yielded stars with significantly lower than eight arms even with low arm<br />
MW. With arm MWs of M n =12,600–19,200 g mol –1 , the number of arms of the<br />
primary stars was only ~5. In addition, higher than expected MW stars were also<br />
obta<strong>in</strong>ed probably by star-star coupl<strong>in</strong>g. 13 C relaxation NMR studies <strong>in</strong>dicated<br />
that the mobility of the arms is not limited by steric compression between them.<br />
Apparently, there is enough room around 27 to place eight arms, although access<br />
to the unreacted Si-H sites may become limited after five to six arms have been<br />
placed.<br />
(26)