142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
142 Advances in Polymer Science Editorial Board: A. Abe. A.-C ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
18 B. Charleux, R. Faust<br />
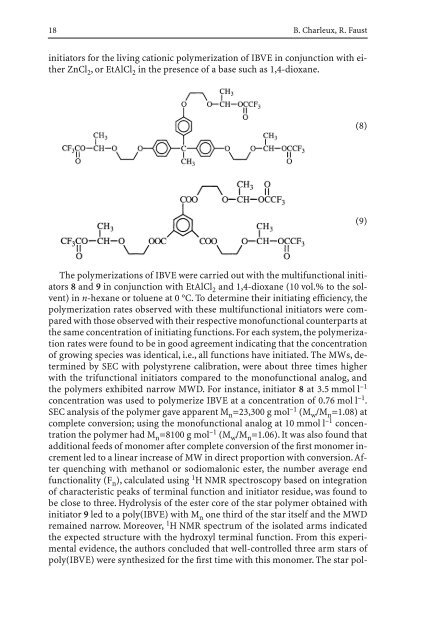
<strong>in</strong>itiators for the liv<strong>in</strong>g cationic polymerization of IBVE <strong>in</strong> conjunction with either<br />
ZnCl 2 , or EtAlCl 2 <strong>in</strong> the presence of a base such as 1,4-dioxane.<br />
The polymerizations of IBVE were carried out with the multifunctional <strong>in</strong>itiators<br />
8 and 9 <strong>in</strong> conjunction with EtAlCl 2 and 1,4-dioxane (10 vol.% to the solvent)<br />
<strong>in</strong> n-hexane or toluene at 0 °C. To determ<strong>in</strong>e their <strong>in</strong>itiat<strong>in</strong>g efficiency, the<br />
polymerization rates observed with these multifunctional <strong>in</strong>itiators were compared<br />
with those observed with their respective monofunctional counterparts at<br />
the same concentration of <strong>in</strong>itiat<strong>in</strong>g functions. For each system, the polymerization<br />
rates were found to be <strong>in</strong> good agreement <strong>in</strong>dicat<strong>in</strong>g that the concentration<br />
of grow<strong>in</strong>g species was identical, i.e., all functions have <strong>in</strong>itiated. The MWs, determ<strong>in</strong>ed<br />
by SEC with polystyrene calibration, were about three times higher<br />
with the trifunctional <strong>in</strong>itiators compared to the monofunctional analog, and<br />
the polymers exhibited narrow MWD. For <strong>in</strong>stance, <strong>in</strong>itiator 8 at 3.5 mmol l –1<br />
concentration was used to polymerize IBVE at a concentration of 0.76 mol l –1 .<br />
SEC analysis of the polymer gave apparent M n =23,300 g mol –1 (M w /M n =1.08) at<br />
complete conversion; us<strong>in</strong>g the monofunctional analog at 10 mmol l –1 concentration<br />
the polymer had M n =8100 g mol –1 (M w /M n =1.06). It was also found that<br />
additional feeds of monomer after complete conversion of the first monomer <strong>in</strong>crement<br />
led to a l<strong>in</strong>ear <strong>in</strong>crease of MW <strong>in</strong> direct proportion with conversion. After<br />
quench<strong>in</strong>g with methanol or sodiomalonic ester, the number average end<br />
functionality (F n), calculated us<strong>in</strong>g 1 H NMR spectroscopy based on <strong>in</strong>tegration<br />
of characteristic peaks of term<strong>in</strong>al function and <strong>in</strong>itiator residue, was found to<br />
be close to three. Hydrolysis of the ester core of the star polymer obta<strong>in</strong>ed with<br />
<strong>in</strong>itiator 9 led to a poly(IBVE) with M n one third of the star itself and the MWD<br />
rema<strong>in</strong>ed narrow. Moreover, 1 H NMR spectrum of the isolated arms <strong>in</strong>dicated<br />
the expected structure with the hydroxyl term<strong>in</strong>al function. From this experimental<br />
evidence, the authors concluded that well-controlled three arm stars of<br />
poly(IBVE) were synthesized for the first time with this monomer. The star pol-<br />
(8)<br />
(9)