Hyperbare Zuurstoftherapie: Rapid Assessment - KCE
Hyperbare Zuurstoftherapie: Rapid Assessment - KCE
Hyperbare Zuurstoftherapie: Rapid Assessment - KCE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>KCE</strong> Reports 74 Hyperbaric Oxygenation Therapy 104<br />
APPENDIX FOR THE CHAPTER ON ECONOMIC<br />
EVALUATION (CHAPTER 4)<br />
SEARCH FOR COST-EFFECTIVENESS STUDIES<br />
In January 2008, the websites of HTA institutes (Table 54) and following databases were<br />
searched: Medline, Embase, Centre for Reviews and Dissemination (CRD) databases<br />
(Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation<br />
Database (NHS EED), and Health Technology <strong>Assessment</strong>s (HTA)), Cochrane Database<br />
of Systematic Reviews (CDSR), and Econlit. In the CDSR database, only the results of<br />
the categories Technology <strong>Assessment</strong>s, Economic Evaluations, and Other Reviews<br />
were retained for this economic search. The following six tables (Table 55 to Table 60)<br />
provide an overview of the search strategy.<br />
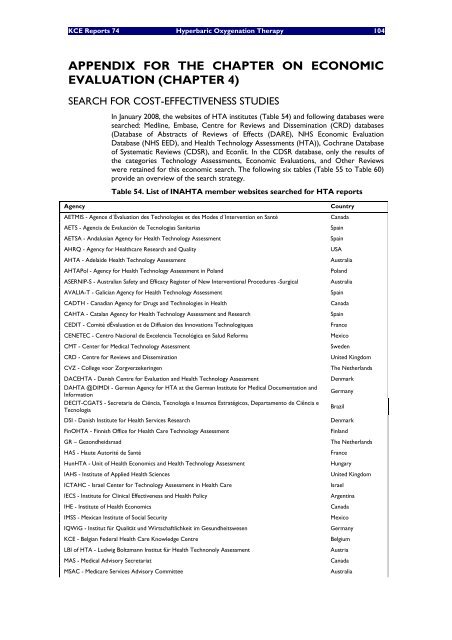
Table 54. List of INAHTA member websites searched for HTA reports<br />
Agency Country<br />
AETMIS - Agence d´Évaluation des Technologies et des Modes d´Intervention en Santé Canada<br />
AETS - Agencia de Evaluación de Tecnologias Sanitarias Spain<br />
AETSA - Andalusian Agency for Health Technology <strong>Assessment</strong> Spain<br />
AHRQ - Agency for Healthcare Research and Quality USA<br />
AHTA - Adelaide Health Technology <strong>Assessment</strong> Australia<br />
AHTAPol - Agency for Health Technology <strong>Assessment</strong> in Poland Poland<br />
ASERNIP-S - Australian Safety and Efficacy Register of New Interventional Procedures -Surgical Australia<br />
AVALIA-T - Galician Agency for Health Technology <strong>Assessment</strong> Spain<br />
CADTH - Canadian Agency for Drugs and Technologies in Health Canada<br />
CAHTA - Catalan Agency for Health Technology <strong>Assessment</strong> and Research Spain<br />
CEDIT - Comité dÉvaluation et de Diffusion des Innovations Technologiques France<br />
CENETEC - Centro Nacional de Excelencia Tecnológica en Salud Reforma Mexico<br />
CMT - Center for Medical Technology <strong>Assessment</strong> Sweden<br />
CRD - Centre for Reviews and Dissemination United Kingdom<br />
CVZ - College voor Zorgverzekeringen The Netherlands<br />
DACEHTA - Danish Centre for Evaluation and Health Technology <strong>Assessment</strong> Denmark<br />
DAHTA @DIMDI - German Agency for HTA at the German Institute for Medical Documentation and<br />
Information<br />
DECIT-CGATS - Secretaria de Ciëncia, Tecnologia e Insumos Estratégicos, Departamento de Ciência e<br />
Tecnologia<br />
Germany<br />
DSI - Danish Institute for Health Services Research Denmark<br />
FinOHTA - Finnish Office for Health Care Technology <strong>Assessment</strong> Finland<br />
GR – Gezondheidsraad The Netherlands<br />
HAS - Haute Autorité de Santé France<br />
HunHTA - Unit of Health Economics and Health Technology <strong>Assessment</strong> Hungary<br />
IAHS - Institute of Applied Health Sciences United Kingdom<br />
ICTAHC - Israel Center for Technology <strong>Assessment</strong> in Health Care Israel<br />
IECS - Institute for Clinical Effectiveness and Health Policy Argentina<br />
IHE - Institute of Health Economics Canada<br />
IMSS - Mexican Institute of Social Security Mexico<br />
IQWiG - Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen Germany<br />
<strong>KCE</strong> - Belgian Federal Health Care Knowledge Centre Belgium<br />
LBI of HTA - Ludwig Boltzmann Institut für Health Technonoly <strong>Assessment</strong> Austria<br />
MAS - Medical Advisory Secretariat Canada<br />
MSAC - Medicare Services Advisory Committee Australia<br />
Brazil