Diploma - Max Planck Institute for Solid State Research
Diploma - Max Planck Institute for Solid State Research
Diploma - Max Planck Institute for Solid State Research
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
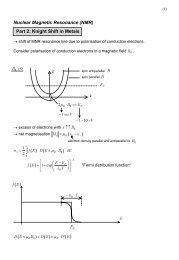
4.2 Hybridization: localized versus itinerant states 43<br />
the energy window, we will get a WF which yields exactly this particular atomic orbital,<br />
because <strong>for</strong> each k-point at least one Kohn-Sham function has a major contribution by<br />
this orbital.<br />
To create a (minimal) WF basis set, one should define molecular orbitals, in this<br />
case mainly hybrids of Eu 4f and Rh 4d as it will be shown later, and check if the WF<br />
Hamiltonian reproduces the band structure inside the chosen energy window. Because<br />
this is a tough task, and we are only interested in the in<strong>for</strong>mation of hybridizing orbitals,<br />
a different approach has been applied. Defining a projector <strong>for</strong> each Eu 4f orbital and<br />
restricting the energy to this particular band of the band structure should define a WF<br />
which consists of the 4f orbital and its symmetry related counterpart at other sites (due<br />
to “hybridization”). This rough procedure is solely suitable, because the dispersion of the<br />
4f dominated bands is very small and all 4f levels can be separated in energy. Internally,<br />
FPLO uses a real representation <strong>for</strong> the complex spherical harmonics of 4f orbitals (the<br />
“general set”). Since the 4f levels are not decoupled (cf fig. 4.14), we use a different<br />
superposition (similar to the “cubic set”) to create a representation so that all orbitals<br />
are well separated by energy and / or symmetry.<br />
In fig. 4.15 the 4f orbitals with respect to the applied projector are depicted, the<br />
corresponding parameters are given in tab. 4.2. In that the energy windows are chosen<br />
arbitrarily, the amount of hybridization cannot be compared between the WFs. Nevertheless,<br />
comparing the WFs in fig. 4.15 the Eu 4f orbitals seem to hybridize primarily<br />
with Rh 4d states despite of the ones in (c) and (e) although silicon is their nearestneighbour.<br />
If the occupation of the 4f orbitals in LSDA+U [AL] reflects the ground<br />
state occupancy, one could restrict the analysis to partially- and entirely-filled orbitals.<br />
But since this scheme does not include spin-orbit coupling and respective excited final<br />
states have mixed occupations of all 4f orbitals, this is not suitable. Regarding tab. 4.2,<br />
one can at least conclude that the Rh 4d z 2 orbital possibly match some contribution to<br />
a hybrid orbital because of its orientation towards the Eu layers and its occurance in<br />
all WFs.<br />
4.2.2 Estimation of the hybridization strength<br />
Depending on the symmetry analysis, one has two possible options to get a first approximation<br />
<strong>for</strong> the interaction strength. If one cannot relate the 4f orbitals with special<br />
linear combinations of Rhodium 4d orbitals by e.g. a basis trans<strong>for</strong>mation or group theory,<br />
then solely a quantative estimation within muffin-tin methods remains. Otherwise<br />
the basis coefficients of the Rh 4d orbitals can be used directly as an estimate <strong>for</strong> the<br />
coupling strength. The first option will be discussed below.<br />
Remembering that in LMTO-ASA no interstitial regions exist, the redistribution of<br />
charge density during the convergence process compared to the initial guess of a superposition<br />
of atomic solutions causes non-vanishing contributions of initially unoccupied<br />
orbitals in the considered sphere as well as in the surrounding ones. Since in our cal-