You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
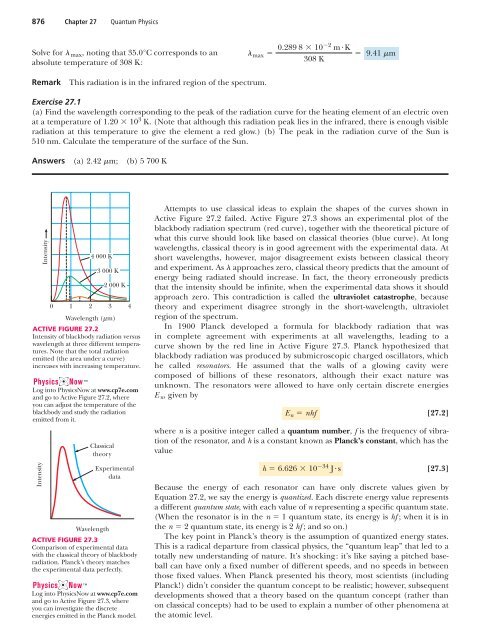
876 Chapter 27 <strong>Quantum</strong> <strong>Physics</strong>Solve for max , noting that 35.0°C corresponds to anabsolute temperature of 308 K:max 0.289 8 102 mK308 K9.41 mRemarkThis radiation is in the infrared region of the spectrum.Exercise 27.1(a) Find the wavelength corresponding to the peak of the radiation curve for the heating element of an electric ovenat a temperature of 1.20 10 3 K. (Note that although this radiation peak lies in the infrared, there is enough visibleradiation at this temperature to give the element a red glow.) (b) The peak in the radiation curve of the Sun is510 nm. Calculate the temperature of the surface of the Sun.Answers (a) 2.42 m; (b) 5 700 KIntensity0124 000 K3 000 K2 000 KWavelength ( µ m)3 4ACTIVE FIGURE 27.2Intensity of blackbody radiation versuswavelength at three different temperatures.Note that the total radiationemitted (the area under a curve)increases with increasing temperature.Log into <strong>Physics</strong>Now at www.cp7e.comand go to Active Figure 27.2, whereyou can adjust the temperature of theblackbody and study the radiationemitted from it.IntensityClassicaltheoryWavelengthExperimentaldataACTIVE FIGURE 27.3Comparison of experimental datawith the classical theory of blackbodyradiation. Planck’s theory matchesthe experimental data perfectly.Log into <strong>Physics</strong>Now at www.cp7e.comand go to Active Figure 27.3, whereyou can investigate the discreteenergies emitted in the Planck model.Attempts to use classical ideas to explain the shapes of the curves shown inActive Figure 27.2 failed. Active Figure 27.3 shows an experimental plot of theblackbody radiation spectrum (red curve), together with the theoretical picture ofwhat this curve should look like based on classical theories (blue curve). At longwavelengths, classical theory is in good agreement with the experimental data. Atshort wavelengths, however, major disagreement exists between classical theoryand experiment. As approaches zero, classical theory predicts that the amount ofenergy being radiated should increase. In fact, the theory erroneously predictsthat the intensity should be infinite, when the experimental data shows it shouldapproach zero. This contradiction is called the ultraviolet catastrophe, becausetheory and experiment disagree strongly in the short-wavelength, ultravioletregion of the spectrum.In 1900 Planck developed a formula for blackbody radiation that wasin complete agreement with experiments at all wavelengths, leading to acurve shown by the red line in Active Figure 27.3. Planck hypothesized thatblackbody radiation was produced by submicroscopic charged oscillators, whichhe called resonators. He assumed that the walls of a glowing cavity werecomposed of billions of these resonators, although their exact nature wasunknown. The resonators were allowed to have only certain discrete energiesE n , given byE n nhf[27.2]where n is a positive integer called a quantum number, f is the frequency of vibrationof the resonator, and h is a constant known as Planck’s constant, which has thevalueh 6.626 10 34 Js[27.3]Because the energy of each resonator can have only discrete values given byEquation 27.2, we say the energy is quantized. Each discrete energy value representsa different quantum state, with each value of n representing a specific quantum state.(When the resonator is in the n 1 quantum state, its energy is hf ; when it is inthe n 2 quantum state, its energy is 2 hf ; and so on.)The key point in Planck’s theory is the assumption of quantized energy states.This is a radical departure from classical physics, the “quantum leap” that led to atotally new understanding of nature. It’s shocking: it’s like saying a pitched baseballcan have only a fixed number of different speeds, and no speeds in betweenthose fixed values. When Planck presented his theory, most scientists (includingPlanck!) didn’t consider the quantum concept to be realistic; however, subsequentdevelopments showed that a theory based on the quantum concept (rather thanon classical concepts) had to be used to explain a number of other phenomena atthe atomic level.