29.3 Radioactivity 94529.3 RADIOACTIVITYIn 1896, Becquerel accidentally discovered that uranium salt crystals emit an invisibleradiation that can darken a photographic plate even if the plate is covered toexclude light. After several such observations under controlled conditions, he concludedthat the radiation emitted by the crystals was of a new type, one requiringno external stimulation. This spontaneous emission of radiation was soon calledradioactivity. Subsequent experiments by other scientists showed that othersubstances were also radioactive.The most significant investigations of this type were conducted by Marieand Pierre Curie. After several years of careful and laborious chemicalseparation processes on tons of pitchblende, a radioactive ore, the Curiesreported the discovery of two previously unknown elements, both of whichwere radioactive. These were named polonium and radium. Subsequent experiments,including Rutherford’s famous work on alpha-particle scattering,suggested that radioactivity was the result of the decay, or disintegration, of unstablenuclei.Three types of radiation can be emitted by a radioactive substance: alpha ()4particles, in which the emitted particles are 2 He nuclei; beta () particles, in whichand with Becquerel for their studies ofradioactive substances. In 1911 she wasMARIE CURIE, Polish Scientist(1867 – 1934)In 1903 Marie Curie shared the NobelPrize in physics with her husband, Pierre,the emitted particles are either electrons or positrons; and gamma () rays, inwhich the emitted “rays” are high-energy photons. A positron is a particle similarto the electron in all respects, except that it has a charge of e. (The positron issaid to be the antiparticle of the electron.) The symbol e is used to designate anawarded a second Nobel Prize in chemistryfor the discovery of radium and polonium.Marie Curie died of leukemia causedby years of exposure to radioactive substances.“I persist in believing that theelectron, and e designates a positron.ideas that then guided us are the onlyIt’s possible to distinguish these three forms of radiation by using the schemeones which can lead to the true socialdescribed in Figure 29.5. The radiation from a radioactive sample is directed intoprogress. We cannot hope to build a bettera region with a magnetic field, and the beam splits into three components, two world without improving the individual.bending in opposite directions and the third not changing direction. From this Toward this end, each of us must worksimple observation it can be concluded that the radiation of the undeflected beam toward his own highest development,(the gamma ray) carries no charge, the component deflected upward containspositively charged particles (alpha particles), and the component deflected downwardcontains negatively charged particles (e ). If the beam includes a positronaccepting at the same time his share ofresponsibility in the general life ofhumanity.”(e ), it is deflected upward.The three types of radiation have quite different penetrating powers. Alphaparticles barely penetrate a sheet of paper, beta particles can penetrate a fewmillimeters of aluminum, and gamma rays can penetrate several centimeters oflead. arrayThe Decay Constant and Half-LifeObservation has shown that if a radioactive sample contains N radioactive nuclei atsome instant, then the number of nuclei, N, that decay in a small time interval tis proportional to N ; mathematically,NNtorN N t [29.2]where is a constant called the decay constant. The negative sign signifies that Ndecreases with time; that is, N is negative. The value of for any isotope determinesthe rate at which that isotope will decay. The decay rate, or activity R, of asample is defined as the number of decays per second. From Equation 29.2, we seethat the decay rate isR Nt NIsotopes with a large value decay rapidly; those with small decay slowly.[29.3]FPG InternationalLeadRadioactivesourceDetector e– B inFigure 29.5 The radiation from aradioactive source, such as radium,can be separated into three componentsusing a magnetic field to deflectthe charged particles. The detectorarray at the right records theevents. The gamma ray isn’t deflectedby the magnetic field. Decay rate
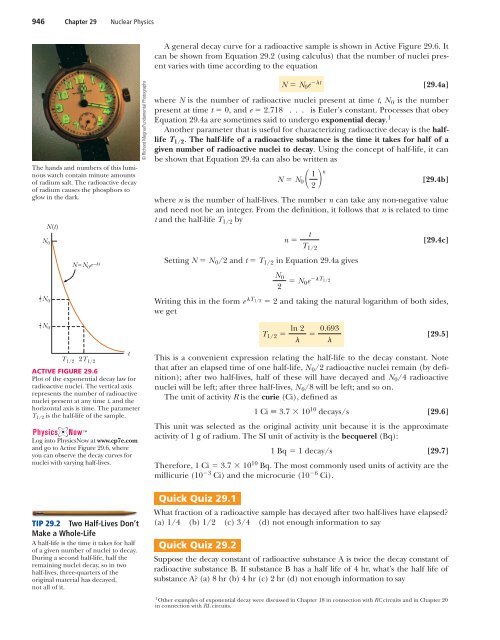
946 Chapter 29 Nuclear <strong>Physics</strong>A general decay curve for a radioactive sample is shown in Active Figure 29.6. Itcan be shown from Equation 29.2 (using calculus) that the number of nuclei presentvaries with time according to the equationThe hands and numbers of this luminouswatch contain minute amountsof radium salt. The radioactive decayof radium causes the phosphors toglow in the dark.N 012 N 014 N 0N(t)T 1/2N =N 0 e – t2T 1/2ACTIVE FIGURE 29.6Plot of the exponential decay law forradioactive nuclei. The vertical axisrepresents the number of radioactivenuclei present at any time t, and thehorizontal axis is time. The parameterT 1/2 is the half-life of the sample.Log into <strong>Physics</strong>Now at www.cp7e.comand go to Active Figure 29.6, whereyou can observe the decay curves fornuclei with varying half-lives.TIP 29.2 Two Half-Lives Don’tMake a Whole-LifeA half-life is the time it takes for halfof a given number of nuclei to decay.During a second half-life, half theremaining nuclei decay, so in twohalf-lives, three-quarters of theoriginal material has decayed,not all of it.t© Richard Megna/Fundamental PhotographsN N 0 e t[29.4a]where N is the number of radioactive nuclei present at time t, N 0 is the numberpresent at time t 0, and e 2.718 . . . is Euler’s constant. Processes that obeyEquation 29.4a are sometimes said to undergo exponential decay. 1Another parameter that is useful for characterizing radioactive decay is the halflifeT 1/2 . The half-life of a radioactive substance is the time it takes for half of agiven number of radioactive nuclei to decay. Using the concept of half-life, it canbe shown that Equation 29.4a can also be written asN N 0 1 2 n[29.4b]where n is the number of half-lives. The number n can take any non-negative valueand need not be an integer. From the definition, it follows that n is related to timet and the half-life T 1/2 byn Setting N N 0 /2 and t T 1/2 in Equation 29.4a givesN 02 N 0e T 1/2[29.4c]Writing this in the form eT 1/2 2 and taking the natural logarithm of both sides,we getT 1/2 ln 2 0.693[29.5]This is a convenient expression relating the half-life to the decay constant. Notethat after an elapsed time of one half-life, N 0 /2 radioactive nuclei remain (by definition);after two half-lives, half of these will have decayed and N 0 /4 radioactivenuclei will be left; after three half-lives, N 0 /8 will be left; and so on.The unit of activity R is the curie (Ci), defined as1 Ci 3.7 10 10 decays/s[29.6]This unit was selected as the original activity unit because it is the approximateactivity of 1 g of radium. The SI unit of activity is the becquerel (Bq):1Bq 1 decay/s [29.7]Therefore, 1 Ci 3.7 10 10 Bq. The most commonly used units of activity are themillicurie (10 3 Ci) and the microcurie (10 6 Ci).Quick Quiz 29.1tT 1/2What fraction of a radioactive sample has decayed after two half-lives have elapsed?(a) 1/4 (b) 1/2 (c) 3/4 (d) not enough information to sayQuick Quiz 29.2Suppose the decay constant of radioactive substance A is twice the decay constant ofradioactive substance B. If substance B has a half life of 4 hr, what’s the half life ofsubstance A? (a) 8 hr (b) 4 hr (c) 2 hr (d) not enough information to say1 Other examples of exponential decay were discussed in Chapter 18 in connection with RC circuits and in Chapter 20in connection with RL circuits.
- Page 1 and 2:
Color-enhanced scanning electronmic
- Page 3:
876 Chapter 27 Quantum PhysicsSolve
- Page 6 and 7:
27.2 The Photoelectric Effect and t
- Page 8 and 9:
27.3 X-Rays 881even when black card
- Page 10 and 11:
27.4 Diffraction of X-Rays by Cryst
- Page 12 and 13:
27.5 The Compton Effect 885Exercise
- Page 14 and 15:
27.6 The Dual Nature of Light and M
- Page 16 and 17:
27.6 The Dual Nature of Light and M
- Page 18 and 19:
27.8 The Uncertainty Principle 891w
- Page 20 and 21:
27.8 The Uncertainty Principle 893E
- Page 22 and 23: 27.9 The Scanning Tunneling Microsc
- Page 24 and 25: Problems 897The probability per uni
- Page 26 and 27: Problems 89917. When light of wavel
- Page 28 and 29: Problems 90151.time of 5.00 ms. Fin
- Page 30 and 31: “Neon lights,” commonly used in
- Page 32 and 33: 28.2 Atomic Spectra 905l(nm) 400 50
- Page 34 and 35: 28.3 The Bohr Theory of Hydrogen 90
- Page 36 and 37: 28.3 Th Bohr Theory of Hydrogen 909
- Page 38 and 39: 28.4 Modification of the Bohr Theor
- Page 40 and 41: 28.6 Quantum Mechanics and the Hydr
- Page 42 and 43: 28.7 The Spin Magnetic Quantum Numb
- Page 44 and 45: 28.9 The Exclusion Principle and th
- Page 46 and 47: 28.9 The Exclusion Principle and th
- Page 48 and 49: 28.11 Atomic Transitions 921electro
- Page 50 and 51: 28.12 Lasers and Holography 923is u
- Page 52 and 53: 28.13 Energy Bands in Solids 925Ene
- Page 54 and 55: 28.13 Energy Bands in Solids 927Ene
- Page 56 and 57: 28.14 Semiconductor Devices 929I (m
- Page 58 and 59: Summary 931(a)Figure 28.32 (a) Jack
- Page 60 and 61: Problems 9335. Is it possible for a
- Page 62 and 63: Problems 935tum number n. (e) Shoul
- Page 64 and 65: Problems 93748. A dimensionless num
- Page 66 and 67: Aerial view of a nuclear power plan
- Page 68 and 69: 29.1 Some Properties of Nuclei 941T
- Page 70 and 71: 29.2 Binding Energy 943130120110100
- Page 74 and 75: 29.3 Radioactivity 947INTERACTIVE E
- Page 76 and 77: 29.4 The Decay Processes 949Alpha D
- Page 78 and 79: 29.4 The Decay Processes 951Strateg
- Page 80 and 81: 29.4 The Decay Processes 953they we
- Page 82 and 83: 29.6 Nuclear Reactions 955wounds on
- Page 84 and 85: 29.6 Nuclear Reactions 957EXAMPLE 2
- Page 86 and 87: 29.7 Medical Applications of Radiat
- Page 88 and 89: 29.7 Medical Applications of Radiat
- Page 90 and 91: 29.8 Radiation Detectors 963Figure
- Page 92 and 93: Summary 965Photo Researchers, Inc./
- Page 94 and 95: Problems 967CONCEPTUAL QUESTIONS1.
- Page 96 and 97: Problems 96924. A building has beco
- Page 98 and 99: Problems 97157. A by-product of som
- Page 100 and 101: This photo shows scientist MelissaD
- Page 102 and 103: 30.1 Nuclear Fission 975Applying Ph
- Page 104 and 105: 30.2 Nuclear Reactors 977Courtesy o
- Page 106 and 107: 30.2 Nuclear Reactors 979events in
- Page 108 and 109: 30.3 Nuclear Fusion 981followed by
- Page 110 and 111: 30.3 Nuclear Fusion 983VacuumCurren
- Page 112 and 113: 30.6 Positrons and Other Antipartic
- Page 114 and 115: 30.7 Mesons and the Beginning of Pa
- Page 116 and 117: 30.9 Conservation Laws 989LeptonsLe
- Page 118 and 119: 30.10 Strange Particles and Strange
- Page 120 and 121: 30.12 Quarks 993n pΣ _ Σ 0 Σ + S
- Page 122 and 123:
30.12 Quarks 995charm C 1, its anti
- Page 124 and 125:
30.14 Electroweak Theory and the St
- Page 126 and 127:
30.15 The Cosmic Connection 999prot
- Page 128 and 129:
30.16 Problems and Perspectives 100
- Page 130 and 131:
Problems 100330.12 Quarks &30.13 Co
- Page 132 and 133:
Problems 1005particles fuse to prod
- Page 134 and 135:
Problems 100740. Assume binding ene
- Page 136 and 137:
A.1 MATHEMATICAL NOTATIONMany mathe
- Page 138 and 139:
A.3 Algebra A.3by 8, we have8x8 32
- Page 140 and 141:
A.3 Algebra A.5EXERCISESSolve the f
- Page 142 and 143:
A.5 Trigonometry A.7When natural lo
- Page 144 and 145:
APPENDIX BAn Abbreviated Table of I
- Page 146 and 147:
An Abbreviated Table of Isotopes A.
- Page 148 and 149:
An Abbreviated Table of Isotopes A.
- Page 150 and 151:
Some Useful Tables A.15TABLE C.3The
- Page 152 and 153:
Answers to Quick Quizzes,Odd-Number
- Page 154 and 155:
Answers to Quick Quizzes, Odd-Numbe
- Page 156 and 157:
Answers to Quick Quizzes, Odd-Numbe
- Page 158 and 159:
Answers to Quick Quizzes, Odd-Numbe
- Page 160 and 161:
Answers to Quick Quizzes, Odd-Numbe
- Page 162 and 163:
Answers to Quick Quizzes, Odd-Numbe
- Page 164 and 165:
Answers to Quick Quizzes, Odd-Numbe
- Page 166 and 167:
Answers to Quick Quizzes, Odd-Numbe
- Page 168 and 169:
IndexPage numbers followed by “f
- Page 170 and 171:
Current, 568-573, 586direction of,
- Page 172 and 173:
Index I.5Fissionnuclear, 973-976, 9
- Page 174 and 175:
Index I.7Magnetic field(s) (Continu
- Page 176 and 177:
Polarizer, 805-806, 805f, 806-807Po
- Page 178 and 179:
South poleEarth’s geographic, 626
- Page 180 and 181:
CreditsPhotographsThis page constit
- Page 182 and 183:
PEDAGOGICAL USE OF COLORDisplacemen
- Page 184 and 185:
PHYSICAL CONSTANTSQuantity Symbol V