28.13 Energy Bands in Solids 925Energy2s 2s 2s1sEnergy1sEnergyEquilibriumseparation1sFigure 28.23 (a) Splitting of the1s and 2s states when two atoms arebrought together. (b) Splitting of the1s and 2s states when five atoms arebrought close together. (c) Formationof energy bands when a large numberof sodium atoms are assembled toform a solid.rrr(a)(b)r 0(c)separated by forbidden gaps. The separation and electron population of thehighest bands determines whether a given solid is a conductor, an insulator, or asemiconductor.Consider two identical atoms, initially widely separated, that are brought closerand closer together. If two identical atoms are very far apart, they do not interact,and their electronic energy levels can be considered to be those of isolated atoms.Hence, the energy levels are exactly the same. As the atoms come close together,they essentially become one quantum system, and the Pauli exclusion principle demandsthat the electrons be in different quantum states for this single system. Theexclusion principle manifests itself as a changing or splitting of electron energylevels that were identical in the widely separated atoms, as shown in Figure 28.23a.Figure 28.23b shows that with 5 atoms, each energy level in the isolated atom splitsinto five different, more closely spaced levels.If we extend this argument to the large number of atoms found in solids (onthe order of 10 23 atoms/cm 3 ), we obtain a large number of levels so closely spacedthat they may be regarded as a continuous band of energy levels, as in Figure28.23c. An electron can have any energy within an allowed energy band, but cannothave an energy in the band gap, or the region between allowed bands. Notethat the band gap energy E g is indicated in Figure 28.23c. In practice we are onlyinterested in the band structure of a solid at some equilibrium separation of itsatoms r 0 , and so we remove the distance scale on the x-axis and simply plot the allowedenergy bands of a solid as a series of horizontal bands, as shown in Figure28.24 for sodium.3pConductors and InsulatorsFigure 28.24 shows that the band structure of a particular solid is quite complicatedwith individual atomic levels broadening by varying amounts and some levels(3s and 3p) broadening so much that they overlap. Nevertheless, it is possible togain a qualitative understanding of whether a solid is a conductor, an insulator, ora semiconductor by considering only the structure of the upper or upper two energybands and whether they are occupied by electrons.Deciding whether an energy band is empty (unoccupied by electrons), partiallyfilled, or full is carried out in basically the same way as for the energy-level populationof atoms: we distribute the total number of electrons from the lowest energylevels up in a way consistent with the exclusion principle. While we omit the detailsof this process here, one important case is that shown in Figure 28.25a (page 926),where the highest-energy occupied band is only partially full. The other importantcase, where the highest occupied band is completely full, is shown in Figure 28.25b.Notice that this figure also shows that the highest filled band is called the valenceband and the next higher empty band is called the conduction band. The energyband gap, which varies with the solid, is also indicated as the energy difference E gbetween the top of the valence band and the bottom of the conduction band.3s2p2s1sFigure 28.24 Energy bands ofsodium. Note the energy gaps (whiteregions) between the allowed bands;electrons can’t occupy states that liein these forbidden gaps. Bluerepresents energy bands occupied bythe sodium electrons when the atomis in its ground state. Gold representsenergy bands that are empty. Notethat the 3s and 3p levels broaden somuch that they overlap.
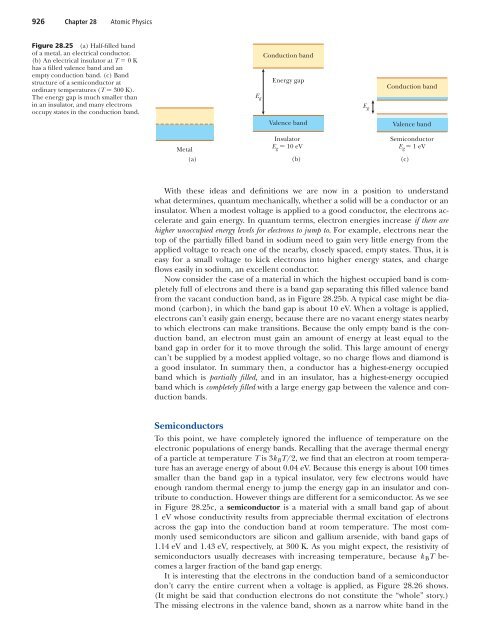
926 Chapter 28 Atomic <strong>Physics</strong>Figure 28.25 (a) Half-filled bandof a metal, an electrical conductor.(b) An electrical insulator at T 0Khas a filled valence band and anempty conduction band. (c) Bandstructure of a semiconductor atordinary temperatures (T 300 K).The energy gap is much smaller thanin an insulator, and many electronsoccupy states in the conduction band.Conduction bandEnergy gapE gE gConduction bandValence bandValence bandMetal(a)InsulatorE g 10 eV(b)SemiconductorE g 1 eV(c)With these ideas and definitions we are now in a position to understandwhat determines, quantum mechanically, whether a solid will be a conductor or aninsulator. When a modest voltage is applied to a good conductor, the electrons accelerateand gain energy. In quantum terms, electron energies increase if there arehigher unoccupied energy levels for electrons to jump to. For example, electrons near thetop of the partially filled band in sodium need to gain very little energy from theapplied voltage to reach one of the nearby, closely spaced, empty states. Thus, it iseasy for a small voltage to kick electrons into higher energy states, and chargeflows easily in sodium, an excellent conductor.Now consider the case of a material in which the highest occupied band is completelyfull of electrons and there is a band gap separating this filled valence bandfrom the vacant conduction band, as in Figure 28.25b. A typical case might be diamond(carbon), in which the band gap is about 10 eV. When a voltage is applied,electrons can’t easily gain energy, because there are no vacant energy states nearbyto which electrons can make transitions. Because the only empty band is the conductionband, an electron must gain an amount of energy at least equal to theband gap in order for it to move through the solid. This large amount of energycan’t be supplied by a modest applied voltage, so no charge flows and diamond isa good insulator. In summary then, a conductor has a highest-energy occupiedband which is partially filled, and in an insulator, has a highest-energy occupiedband which is completely filled with a large energy gap between the valence and conductionbands.SemiconductorsTo this point, we have completely ignored the influence of temperature on theelectronic populations of energy bands. Recalling that the average thermal energyof a particle at temperature T is 3k B T/2, we find that an electron at room temperaturehas an average energy of about 0.04 eV. Because this energy is about 100 timessmaller than the band gap in a typical insulator, very few electrons would haveenough random thermal energy to jump the energy gap in an insulator and contributeto conduction. However things are different for a semiconductor. As we seein Figure 28.25c, a semiconductor is a material with a small band gap of about1 eV whose conductivity results from appreciable thermal excitation of electronsacross the gap into the conduction band at room temperature. The most commonlyused semiconductors are silicon and gallium arsenide, with band gaps of1.14 eV and 1.43 eV, respectively, at 300 K. As you might expect, the resistivity ofsemiconductors usually decreases with increasing temperature, because k B T becomesa larger fraction of the band gap energy.It is interesting that the electrons in the conduction band of a semiconductordon’t carry the entire current when a voltage is applied, as Figure 28.26 shows.(It might be said that conduction electrons do not constitute the “whole” story.)The missing electrons in the valence band, shown as a narrow white band in the
- Page 1 and 2: Color-enhanced scanning electronmic
- Page 3: 876 Chapter 27 Quantum PhysicsSolve
- Page 6 and 7: 27.2 The Photoelectric Effect and t
- Page 8 and 9: 27.3 X-Rays 881even when black card
- Page 10 and 11: 27.4 Diffraction of X-Rays by Cryst
- Page 12 and 13: 27.5 The Compton Effect 885Exercise
- Page 14 and 15: 27.6 The Dual Nature of Light and M
- Page 16 and 17: 27.6 The Dual Nature of Light and M
- Page 18 and 19: 27.8 The Uncertainty Principle 891w
- Page 20 and 21: 27.8 The Uncertainty Principle 893E
- Page 22 and 23: 27.9 The Scanning Tunneling Microsc
- Page 24 and 25: Problems 897The probability per uni
- Page 26 and 27: Problems 89917. When light of wavel
- Page 28 and 29: Problems 90151.time of 5.00 ms. Fin
- Page 30 and 31: “Neon lights,” commonly used in
- Page 32 and 33: 28.2 Atomic Spectra 905l(nm) 400 50
- Page 34 and 35: 28.3 The Bohr Theory of Hydrogen 90
- Page 36 and 37: 28.3 Th Bohr Theory of Hydrogen 909
- Page 38 and 39: 28.4 Modification of the Bohr Theor
- Page 40 and 41: 28.6 Quantum Mechanics and the Hydr
- Page 42 and 43: 28.7 The Spin Magnetic Quantum Numb
- Page 44 and 45: 28.9 The Exclusion Principle and th
- Page 46 and 47: 28.9 The Exclusion Principle and th
- Page 48 and 49: 28.11 Atomic Transitions 921electro
- Page 50 and 51: 28.12 Lasers and Holography 923is u
- Page 54 and 55: 28.13 Energy Bands in Solids 927Ene
- Page 56 and 57: 28.14 Semiconductor Devices 929I (m
- Page 58 and 59: Summary 931(a)Figure 28.32 (a) Jack
- Page 60 and 61: Problems 9335. Is it possible for a
- Page 62 and 63: Problems 935tum number n. (e) Shoul
- Page 64 and 65: Problems 93748. A dimensionless num
- Page 66 and 67: Aerial view of a nuclear power plan
- Page 68 and 69: 29.1 Some Properties of Nuclei 941T
- Page 70 and 71: 29.2 Binding Energy 943130120110100
- Page 72 and 73: 29.3 Radioactivity 94529.3 RADIOACT
- Page 74 and 75: 29.3 Radioactivity 947INTERACTIVE E
- Page 76 and 77: 29.4 The Decay Processes 949Alpha D
- Page 78 and 79: 29.4 The Decay Processes 951Strateg
- Page 80 and 81: 29.4 The Decay Processes 953they we
- Page 82 and 83: 29.6 Nuclear Reactions 955wounds on
- Page 84 and 85: 29.6 Nuclear Reactions 957EXAMPLE 2
- Page 86 and 87: 29.7 Medical Applications of Radiat
- Page 88 and 89: 29.7 Medical Applications of Radiat
- Page 90 and 91: 29.8 Radiation Detectors 963Figure
- Page 92 and 93: Summary 965Photo Researchers, Inc./
- Page 94 and 95: Problems 967CONCEPTUAL QUESTIONS1.
- Page 96 and 97: Problems 96924. A building has beco
- Page 98 and 99: Problems 97157. A by-product of som
- Page 100 and 101: This photo shows scientist MelissaD
- Page 102 and 103:
30.1 Nuclear Fission 975Applying Ph
- Page 104 and 105:
30.2 Nuclear Reactors 977Courtesy o
- Page 106 and 107:
30.2 Nuclear Reactors 979events in
- Page 108 and 109:
30.3 Nuclear Fusion 981followed by
- Page 110 and 111:
30.3 Nuclear Fusion 983VacuumCurren
- Page 112 and 113:
30.6 Positrons and Other Antipartic
- Page 114 and 115:
30.7 Mesons and the Beginning of Pa
- Page 116 and 117:
30.9 Conservation Laws 989LeptonsLe
- Page 118 and 119:
30.10 Strange Particles and Strange
- Page 120 and 121:
30.12 Quarks 993n pΣ _ Σ 0 Σ + S
- Page 122 and 123:
30.12 Quarks 995charm C 1, its anti
- Page 124 and 125:
30.14 Electroweak Theory and the St
- Page 126 and 127:
30.15 The Cosmic Connection 999prot
- Page 128 and 129:
30.16 Problems and Perspectives 100
- Page 130 and 131:
Problems 100330.12 Quarks &30.13 Co
- Page 132 and 133:
Problems 1005particles fuse to prod
- Page 134 and 135:
Problems 100740. Assume binding ene
- Page 136 and 137:
A.1 MATHEMATICAL NOTATIONMany mathe
- Page 138 and 139:
A.3 Algebra A.3by 8, we have8x8 32
- Page 140 and 141:
A.3 Algebra A.5EXERCISESSolve the f
- Page 142 and 143:
A.5 Trigonometry A.7When natural lo
- Page 144 and 145:
APPENDIX BAn Abbreviated Table of I
- Page 146 and 147:
An Abbreviated Table of Isotopes A.
- Page 148 and 149:
An Abbreviated Table of Isotopes A.
- Page 150 and 151:
Some Useful Tables A.15TABLE C.3The
- Page 152 and 153:
Answers to Quick Quizzes,Odd-Number
- Page 154 and 155:
Answers to Quick Quizzes, Odd-Numbe
- Page 156 and 157:
Answers to Quick Quizzes, Odd-Numbe
- Page 158 and 159:
Answers to Quick Quizzes, Odd-Numbe
- Page 160 and 161:
Answers to Quick Quizzes, Odd-Numbe
- Page 162 and 163:
Answers to Quick Quizzes, Odd-Numbe
- Page 164 and 165:
Answers to Quick Quizzes, Odd-Numbe
- Page 166 and 167:
Answers to Quick Quizzes, Odd-Numbe
- Page 168 and 169:
IndexPage numbers followed by “f
- Page 170 and 171:
Current, 568-573, 586direction of,
- Page 172 and 173:
Index I.5Fissionnuclear, 973-976, 9
- Page 174 and 175:
Index I.7Magnetic field(s) (Continu
- Page 176 and 177:
Polarizer, 805-806, 805f, 806-807Po
- Page 178 and 179:
South poleEarth’s geographic, 626
- Page 180 and 181:
CreditsPhotographsThis page constit
- Page 182 and 183:
PEDAGOGICAL USE OF COLORDisplacemen
- Page 184 and 185:
PHYSICAL CONSTANTSQuantity Symbol V