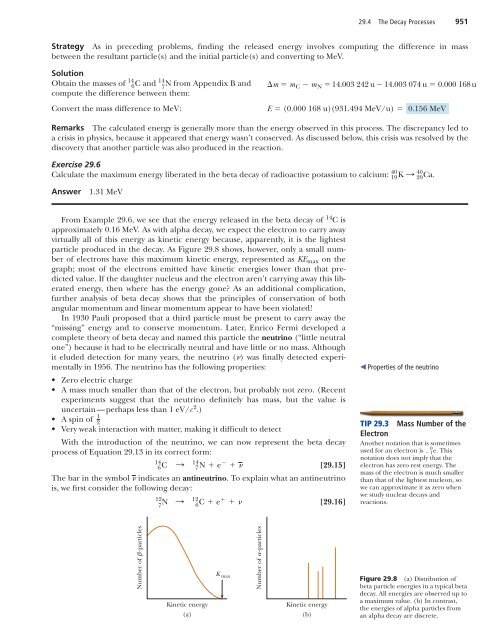
29.4 The Decay Processes 951Strategy As in preceding problems, finding the released energy involves computing the difference in massbetween the resultant particle(s) and the initial particle(s) and converting to MeV.Solution14 14Obtain the masses of 6 C and 7N from Appendix B and m m C m N 14.003 242 u 14.003 074 u 0.000 168 ucompute the difference between them:Convert the mass difference to MeV:E (0.000 168 u)(931.494 MeV/u) 0.156 MeVRemarks The calculated energy is generally more than the energy observed in this process. The discrepancy led toa crisis in physics, because it appeared that energy wasn’t conserved. As discussed below, this crisis was resolved by thediscovery that another particle was also produced in the reaction.Exercise 29.640Calculate the maximum energy liberated in the beta decay of radioactive potassium to calcium: 19 K : 4020 Ca .Answer1.31 MeVFrom Example 29.6, we see that the energy released in the beta decay of 14 C isapproximately 0.16 MeV. As with alpha decay, we expect the electron to carry awayvirtually all of this energy as kinetic energy because, apparently, it is the lightestparticle produced in the decay. As Figure 29.8 shows, however, only a small numberof electrons have this maximum kinetic energy, represented as KE max on thegraph; most of the electrons emitted have kinetic energies lower than that predictedvalue. If the daughter nucleus and the electron aren’t carrying away this liberatedenergy, then where has the energy gone? As an additional complication,further analysis of beta decay shows that the principles of conservation of bothangular momentum and linear momentum appear to have been violated!In 1930 Pauli proposed that a third particle must be present to carry away the“missing” energy and to conserve momentum. Later, Enrico Fermi developed acomplete theory of beta decay and named this particle the neutrino (“little neutralone”) because it had to be electrically neutral and have little or no mass. Althoughit eluded detection for many years, the neutrino () was finally detected experimentallyin 1956. The neutrino has the following properties:• Zero electric charge• A mass much smaller than that of the electron, but probably not zero. (Recentexperiments suggest that the neutrino definitely has mass, but the value isuncertain—perhaps less than 1 eV/c 2 .)1• A spin of2• Very weak interaction with matter, making it difficult to detectWith the introduction of the neutrino, we can now represent the beta decayprocess of Equation 29.13 in its correct form:146 C : 14 7 N e [29.15]The bar in the symbol indicates an antineutrino. To explain what an antineutrinois, we first consider the following decay:127N : 12 6 C e [29.16] Properties of the neutrinoTIP 29.3 Mass Number of theElectronAnother notation that is sometimes0used for an electron is 1 e . Thisnotation does not imply that theelectron has zero rest energy. Themass of the electron is much smallerthan that of the lightest nucleon, sowe can approximate it as zero whenwe study nuclear decays andreactions.Number of -particlesKinetic energy(a)K maxNumber of -particlesKinetic energy(b)Figure 29.8 (a) Distribution ofbeta particle energies in a typical betadecay. All energies are observed up toa maximum value. (b) In contrast,the energies of alpha particles froman alpha decay are discrete.
952 Chapter 29 Nuclear <strong>Physics</strong>Here, we see that when 12 N decays into 12 C, a particle is produced which is identicalto the electron except that it has a positive charge of e. This particle is calleda positron. Because it is like the electron in all respects except charge, the positronis said to be the antiparticle of the electron. We will discuss antiparticles further inChapter 30; for now, it suffices to say that, in beta decay, an electron and an antineutrinoare emitted or a positron and a neutrino are emitted.Unlike beta decay, which results in a daughter particle with a variety of possiblekinetic energies, alpha decays come in discrete amounts, as seen in Figure 29.8b.This is because the two daughter particles have momenta with equal magnitudeand opposite direction and are each composed of a fixed number of nucleons.ENRICO FERMI, Italian Physicist(1901–1954)Fermi was awarded the Nobel Prize in1938 for producing the transuranicelements by neutron irradiation and forhis discovery of nuclear reactions boughtabout by slow neutrons. He made manyother outstanding contributions to physics,including his theory of beta decay, thefree-electron theory of metals, and thedevelopment of the world’s first fissionreactor in 1942. Fermi was truly a giftedtheoretical and experimental physicist. Hewas also well known for his ability topresent physics in a clear and excitingmanner. “Whatever Nature has in store formankind, unpleasant as it may be, menmust accept, for ignorance is never betterthan knowledge.”APPLICATIONCarbon Dating of theDead Sea ScrollsNational Accelerator LaboratoryGamma DecayVery often a nucleus that undergoes radioactive decay is left in an excited energystate. The nucleus can then undergo a second decay to a lower energy state—perhaps even to the ground state—by emitting one or more high-energy photons.The process is similar to the emission of light by an atom. An atom emits radiationto release some extra energy when an electron “jumps” from a state of high energyto a state of lower energy. Likewise, the nucleus uses essentially the same methodto release any extra energy it may have following a decay or some other nuclearevent. In nuclear de-excitation, the “jumps” that release energy are made by protonsor neutrons in the nucleus as they move from a higher energy level to a lowerlevel. The photons emitted in the process are called gamma rays, which have veryhigh energy relative to the energy of visible light.A nucleus may reach an excited state as the result of a violent collision withanother particle. However, it’s more common for a nucleus to be in an excitedstate as a result of alpha or beta decay. The following sequence of events typifiesthe gamma decay processes:125 B : 12 6C * e [29.17]126 C * 12: 6 C [29.18]Equation 29.17 represents a beta decay in which 12 B decays to 12 C * , where theasterisk indicates that the carbon nucleus is left in an excited state following thedecay. The excited carbon nucleus then decays to the ground state by emitting agamma ray, as indicated by Equation 29.18. Note that gamma emission doesn’tresult in any change in either Z or A.Practical Uses of RadioactivityCarbon Dating The beta decay of 14 C given by Equation 29.15 is commonly usedto date organic samples. Cosmic rays (high-energy particles from outer space) inthe upper atmosphere cause nuclear reactions that create 14 C from 14 N. In fact,the ratio of 14 C to 12 C (by numbers of nuclei) in the carbon dioxide molecules ofour atmosphere has a constant value of about 1.3 10 12 , as determined by measuringcarbon ratios in tree rings. All living organisms have the same ratio of 14 Cto 12 C because they continuously exchange carbon dioxide with their surroundings.When an organism dies, however, it no longer absorbs 14 C from the atmosphere,so the ratio of 14 C to 12 C decreases as the result of the beta decay of 14 C. It’stherefore possible to determine the age of a material by measuring its activity perunit mass as a result of the decay of 14 C. Through carbon dating, samples of wood,charcoal, bone, and shell have been identified as having lived from 1 000 to 25 000years ago. This knowledge has helped researchers reconstruct the history of livingorganism—including human—during that time span.A particularly interesting example is the dating of the Dead Sea Scrolls. Thisgroup of manuscripts was first discovered by a young Bedouin boy in a cave atQumran near the Dead Sea in 1947. Translation showed the manuscripts to bereligious documents, including most of the books of the Old Testament. Becauseof their historical and religious significance, scholars wanted to know their age.Carbon dating applied to fragments of the scrolls and to the material in which
- Page 1 and 2:
Color-enhanced scanning electronmic
- Page 3:
876 Chapter 27 Quantum PhysicsSolve
- Page 6 and 7:
27.2 The Photoelectric Effect and t
- Page 8 and 9:
27.3 X-Rays 881even when black card
- Page 10 and 11:
27.4 Diffraction of X-Rays by Cryst
- Page 12 and 13:
27.5 The Compton Effect 885Exercise
- Page 14 and 15:
27.6 The Dual Nature of Light and M
- Page 16 and 17:
27.6 The Dual Nature of Light and M
- Page 18 and 19:
27.8 The Uncertainty Principle 891w
- Page 20 and 21:
27.8 The Uncertainty Principle 893E
- Page 22 and 23:
27.9 The Scanning Tunneling Microsc
- Page 24 and 25:
Problems 897The probability per uni
- Page 26 and 27:
Problems 89917. When light of wavel
- Page 28 and 29: Problems 90151.time of 5.00 ms. Fin
- Page 30 and 31: “Neon lights,” commonly used in
- Page 32 and 33: 28.2 Atomic Spectra 905l(nm) 400 50
- Page 34 and 35: 28.3 The Bohr Theory of Hydrogen 90
- Page 36 and 37: 28.3 Th Bohr Theory of Hydrogen 909
- Page 38 and 39: 28.4 Modification of the Bohr Theor
- Page 40 and 41: 28.6 Quantum Mechanics and the Hydr
- Page 42 and 43: 28.7 The Spin Magnetic Quantum Numb
- Page 44 and 45: 28.9 The Exclusion Principle and th
- Page 46 and 47: 28.9 The Exclusion Principle and th
- Page 48 and 49: 28.11 Atomic Transitions 921electro
- Page 50 and 51: 28.12 Lasers and Holography 923is u
- Page 52 and 53: 28.13 Energy Bands in Solids 925Ene
- Page 54 and 55: 28.13 Energy Bands in Solids 927Ene
- Page 56 and 57: 28.14 Semiconductor Devices 929I (m
- Page 58 and 59: Summary 931(a)Figure 28.32 (a) Jack
- Page 60 and 61: Problems 9335. Is it possible for a
- Page 62 and 63: Problems 935tum number n. (e) Shoul
- Page 64 and 65: Problems 93748. A dimensionless num
- Page 66 and 67: Aerial view of a nuclear power plan
- Page 68 and 69: 29.1 Some Properties of Nuclei 941T
- Page 70 and 71: 29.2 Binding Energy 943130120110100
- Page 72 and 73: 29.3 Radioactivity 94529.3 RADIOACT
- Page 74 and 75: 29.3 Radioactivity 947INTERACTIVE E
- Page 76 and 77: 29.4 The Decay Processes 949Alpha D
- Page 80 and 81: 29.4 The Decay Processes 953they we
- Page 82 and 83: 29.6 Nuclear Reactions 955wounds on
- Page 84 and 85: 29.6 Nuclear Reactions 957EXAMPLE 2
- Page 86 and 87: 29.7 Medical Applications of Radiat
- Page 88 and 89: 29.7 Medical Applications of Radiat
- Page 90 and 91: 29.8 Radiation Detectors 963Figure
- Page 92 and 93: Summary 965Photo Researchers, Inc./
- Page 94 and 95: Problems 967CONCEPTUAL QUESTIONS1.
- Page 96 and 97: Problems 96924. A building has beco
- Page 98 and 99: Problems 97157. A by-product of som
- Page 100 and 101: This photo shows scientist MelissaD
- Page 102 and 103: 30.1 Nuclear Fission 975Applying Ph
- Page 104 and 105: 30.2 Nuclear Reactors 977Courtesy o
- Page 106 and 107: 30.2 Nuclear Reactors 979events in
- Page 108 and 109: 30.3 Nuclear Fusion 981followed by
- Page 110 and 111: 30.3 Nuclear Fusion 983VacuumCurren
- Page 112 and 113: 30.6 Positrons and Other Antipartic
- Page 114 and 115: 30.7 Mesons and the Beginning of Pa
- Page 116 and 117: 30.9 Conservation Laws 989LeptonsLe
- Page 118 and 119: 30.10 Strange Particles and Strange
- Page 120 and 121: 30.12 Quarks 993n pΣ _ Σ 0 Σ + S
- Page 122 and 123: 30.12 Quarks 995charm C 1, its anti
- Page 124 and 125: 30.14 Electroweak Theory and the St
- Page 126 and 127: 30.15 The Cosmic Connection 999prot
- Page 128 and 129:
30.16 Problems and Perspectives 100
- Page 130 and 131:
Problems 100330.12 Quarks &30.13 Co
- Page 132 and 133:
Problems 1005particles fuse to prod
- Page 134 and 135:
Problems 100740. Assume binding ene
- Page 136 and 137:
A.1 MATHEMATICAL NOTATIONMany mathe
- Page 138 and 139:
A.3 Algebra A.3by 8, we have8x8 32
- Page 140 and 141:
A.3 Algebra A.5EXERCISESSolve the f
- Page 142 and 143:
A.5 Trigonometry A.7When natural lo
- Page 144 and 145:
APPENDIX BAn Abbreviated Table of I
- Page 146 and 147:
An Abbreviated Table of Isotopes A.
- Page 148 and 149:
An Abbreviated Table of Isotopes A.
- Page 150 and 151:
Some Useful Tables A.15TABLE C.3The
- Page 152 and 153:
Answers to Quick Quizzes,Odd-Number
- Page 154 and 155:
Answers to Quick Quizzes, Odd-Numbe
- Page 156 and 157:
Answers to Quick Quizzes, Odd-Numbe
- Page 158 and 159:
Answers to Quick Quizzes, Odd-Numbe
- Page 160 and 161:
Answers to Quick Quizzes, Odd-Numbe
- Page 162 and 163:
Answers to Quick Quizzes, Odd-Numbe
- Page 164 and 165:
Answers to Quick Quizzes, Odd-Numbe
- Page 166 and 167:
Answers to Quick Quizzes, Odd-Numbe
- Page 168 and 169:
IndexPage numbers followed by “f
- Page 170 and 171:
Current, 568-573, 586direction of,
- Page 172 and 173:
Index I.5Fissionnuclear, 973-976, 9
- Page 174 and 175:
Index I.7Magnetic field(s) (Continu
- Page 176 and 177:
Polarizer, 805-806, 805f, 806-807Po
- Page 178 and 179:
South poleEarth’s geographic, 626
- Page 180 and 181:
CreditsPhotographsThis page constit
- Page 182 and 183:
PEDAGOGICAL USE OF COLORDisplacemen
- Page 184 and 185:
PHYSICAL CONSTANTSQuantity Symbol V