Premenstrual Syndromes : PMS and PMDD - Rutuja :: The site ...
Premenstrual Syndromes : PMS and PMDD - Rutuja :: The site ...
Premenstrual Syndromes : PMS and PMDD - Rutuja :: The site ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
104 THE PREMENSTRUAL SYNDROMES<br />
GABA constitute more than 90% of cortical neurons in<br />
the adult mammalian brain 39 <strong>and</strong> are highly sensitive to<br />
sex hormones <strong>and</strong> neurosteroids.<br />
Before reviewing the literature demonstrating altered<br />
GABAergic function in women with <strong>PMS</strong>/<strong>PMDD</strong>, it is<br />
important to consider the synthetic relationship<br />
between glutamate <strong>and</strong> GABA. In addition, glial cells,<br />
which were once simply thought to support neuronal<br />
function, are also sensitive to sex hormones <strong>and</strong> play a<br />
critical role in glutamate <strong>and</strong> GABA uptake from the<br />
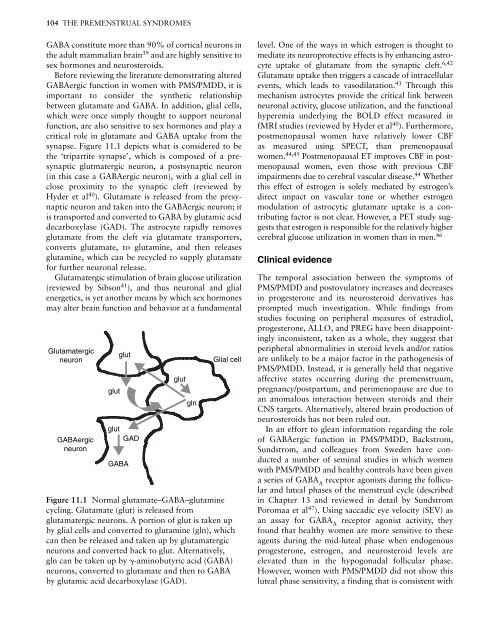
synapse. Figure 11.1 depicts what is considered to be<br />
the ‘tripartite synapse’, which is composed of a presynaptic<br />
glutmatergic neuron, a postsynaptic neuron<br />
(in this case a GABAergic neuron), with a glial cell in<br />
close proximity to the synaptic cleft (reviewed by<br />
Hyder et al 40 ). Glutamate is released from the presynaptic<br />
neuron <strong>and</strong> taken into the GABAergic neuron; it<br />
is transported <strong>and</strong> converted to GABA by glutamic acid<br />
decarboxylase (GAD). <strong>The</strong> astrocyte rapidly removes<br />
glutamate from the cleft via glutamate transporters,<br />
converts glutamate, to glutamine, <strong>and</strong> then releases<br />
glutamine, which can be recycled to supply glutamate<br />
for further neuronal release.<br />
Glutamatergic stimulation of brain glucose utilization<br />
(reviewed by Sibson 41 ), <strong>and</strong> thus neuronal <strong>and</strong> glial<br />
energetics, is yet another means by which sex hormones<br />
may alter brain function <strong>and</strong> behavior at a fundamental<br />
Glutamatergic<br />
glut<br />
neuron Glial cell<br />
GABAergic<br />
neuron<br />
glut<br />
glut<br />
GAD<br />
GABA<br />
glut<br />
gln<br />
Figure 11.1 Normal glutamate–GABA–glutamine<br />
cycling. Glutamate (glut) is released from<br />
glutamatergic neurons. A portion of glut is taken up<br />
by glial cells <strong>and</strong> converted to glutamine (gln), which<br />
can then be released <strong>and</strong> taken up by glutamatergic<br />
neurons <strong>and</strong> converted back to glut. Alternatively,<br />
gln can be taken up by �-aminobutyric acid (GABA)<br />
neurons, converted to glutamate <strong>and</strong> then to GABA<br />
by glutamic acid decarboxylase (GAD).<br />
level. One of the ways in which estrogen is thought to<br />
mediate its neuroprotective effects is by enhancing astrocyte<br />
uptake of glutamate from the synaptic cleft. 6,42<br />
Glutamate uptake then triggers a cascade of intracellular<br />
events, which leads to vasodilatation. 43 Through this<br />
mechanism astrocytes provide the critical link between<br />
neuronal activity, glucose utilization, <strong>and</strong> the functional<br />
hyperemia underlying the BOLD effect measured in<br />
fMRI studies (reviewed by Hyder et al 40 ). Furthermore,<br />
postmenopausal women have relatively lower CBF<br />
as measured using SPECT, than premenopausal<br />
women. 44,45 Postmenopausal ET improves CBF in postmenopausal<br />
women, even those with previous CBF<br />
impairments due to cerebral vascular disease. 44 Whether<br />
this effect of estrogen is solely mediated by estrogen’s<br />
direct impact on vascular tone or whether estrogen<br />
modulation of astrocytic glutamate uptake is a contributing<br />
factor is not clear. However, a PET study suggests<br />
that estrogen is responsible for the relatively higher<br />
cerebral glucose utilization in women than in men. 46<br />
Clinical evidence<br />
<strong>The</strong> temporal association between the symptoms of<br />
<strong>PMS</strong>/<strong>PMDD</strong> <strong>and</strong> postovulatory increases <strong>and</strong> decreases<br />
in progesterone <strong>and</strong> its neurosteroid derivatives has<br />
prompted much investigation. While findings from<br />
studies focusing on peripheral measures of estradiol,<br />
progesterone, ALLO, <strong>and</strong> PREG have been disappointingly<br />
inconsistent, taken as a whole, they suggest that<br />
peripheral abnormalities in steroid levels <strong>and</strong>/or ratios<br />
are unlikely to be a major factor in the pathogenesis of<br />
<strong>PMS</strong>/<strong>PMDD</strong>. Instead, it is generally held that negative<br />
affective states occurring during the premenstruum,<br />
pregnancy/postpartum, <strong>and</strong> perimenopause are due to<br />
an anomalous interaction between steroids <strong>and</strong> their<br />
CNS targets. Alternatively, altered brain production of<br />
neurosteroids has not been ruled out.<br />
In an effort to glean information regarding the role<br />
of GABAergic function in <strong>PMS</strong>/<strong>PMDD</strong>, Backstrom,<br />
Sundstrom, <strong>and</strong> colleagues from Sweden have conducted<br />
a number of seminal studies in which women<br />
with <strong>PMS</strong>/<strong>PMDD</strong> <strong>and</strong> healthy controls have been given<br />
a series of GABA A receptor agonists during the follicular<br />
<strong>and</strong> luteal phases of the menstrual cycle (described<br />
in Chapter 13 <strong>and</strong> reviewed in detail by Sundstrom<br />
Poromaa et al 47 ). Using saccadic eye velocity (SEV) as<br />
an assay for GABA A receptor agonist activity, they<br />
found that healthy women are more sensitive to these<br />
agents during the mid-luteal phase when endogenous<br />
progesterone, estrogen, <strong>and</strong> neurosteroid levels are<br />
elevated than in the hypogonadal follicular phase.<br />
However, women with <strong>PMS</strong>/<strong>PMDD</strong> did not show this<br />
luteal phase sensitivity, a finding that is consistent with