Premenstrual Syndromes : PMS and PMDD - Rutuja :: The site ...
Premenstrual Syndromes : PMS and PMDD - Rutuja :: The site ...
Premenstrual Syndromes : PMS and PMDD - Rutuja :: The site ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
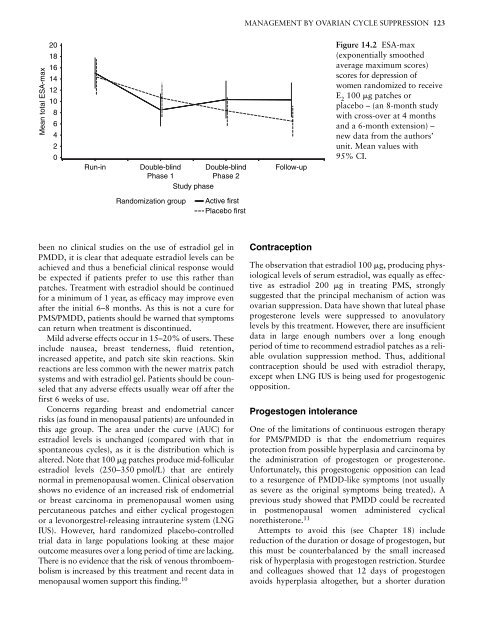
Mean total ESA-max<br />
20<br />
18<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
Run-in<br />
Double-blind Double-blind<br />
Phase 1<br />
Phase 2<br />
Study phase<br />
R<strong>and</strong>omization group Active first<br />
Placebo first<br />
been no clinical studies on the use of estradiol gel in<br />
<strong>PMDD</strong>, it is clear that adequate estradiol levels can be<br />
achieved <strong>and</strong> thus a beneficial clinical response would<br />
be expected if patients prefer to use this rather than<br />
patches. Treatment with estradiol should be continued<br />
for a minimum of 1 year, as efficacy may improve even<br />
after the initial 6–8 months. As this is not a cure for<br />
<strong>PMS</strong>/<strong>PMDD</strong>, patients should be warned that symptoms<br />
can return when treatment is discontinued.<br />
Mild adverse effects occur in 15–20% of users. <strong>The</strong>se<br />
include nausea, breast tenderness, fluid retention,<br />
increased appetite, <strong>and</strong> patch <strong>site</strong> skin reactions. Skin<br />
reactions are less common with the newer matrix patch<br />
systems <strong>and</strong> with estradiol gel. Patients should be counseled<br />
that any adverse effects usually wear off after the<br />
first 6 weeks of use.<br />
Concerns regarding breast <strong>and</strong> endometrial cancer<br />
risks (as found in menopausal patients) are unfounded in<br />
this age group. <strong>The</strong> area under the curve (AUC) for<br />
estradiol levels is unchanged (compared with that in<br />
spontaneous cycles), as it is the distribution which is<br />
altered. Note that 100 �g patches produce mid-follicular<br />
estradiol levels (250–350 pmol/L) that are entirely<br />
normal in premenopausal women. Clinical observation<br />
shows no evidence of an increased risk of endometrial<br />
or breast carcinoma in premenopausal women using<br />
percutaneous patches <strong>and</strong> either cyclical progestogen<br />
or a levonorgestrel-releasing intrauterine system (LNG<br />
IUS). However, hard r<strong>and</strong>omized placebo-controlled<br />
trial data in large populations looking at these major<br />
outcome measures over a long period of time are lacking.<br />
<strong>The</strong>re is no evidence that the risk of venous thromboembolism<br />
is increased by this treatment <strong>and</strong> recent data in<br />
menopausal women support this finding. 10<br />
MANAGEMENT BY OVARIAN CYCLE SUPPRESSION 123<br />
Follow-up<br />
Contraception<br />
<strong>The</strong> observation that estradiol 100 �g, producing physiological<br />
levels of serum estradiol, was equally as effective<br />
as estradiol 200 �g in treating <strong>PMS</strong>, strongly<br />
suggested that the principal mechanism of action was<br />
ovarian suppression. Data have shown that luteal phase<br />
progesterone levels were suppressed to anovulatory<br />
levels by this treatment. However, there are insufficient<br />
data in large enough numbers over a long enough<br />
period of time to recommend estradiol patches as a reliable<br />
ovulation suppression method. Thus, additional<br />
contraception should be used with estradiol therapy,<br />
except when LNG IUS is being used for progestogenic<br />
opposition.<br />
Progestogen intolerance<br />
Figure 14.2 ESA-max<br />
(exponentially smoothed<br />
average maximum scores)<br />
scores for depression of<br />
women r<strong>and</strong>omized to receive<br />
E 2 100 �g patches or<br />
placebo – (an 8-month study<br />
with cross-over at 4 months<br />
<strong>and</strong> a 6-month extension) –<br />
new data from the authors’<br />
unit. Mean values with<br />
95% CI.<br />
One of the limitations of continuous estrogen therapy<br />
for <strong>PMS</strong>/<strong>PMDD</strong> is that the endometrium requires<br />
protection from possible hyperplasia <strong>and</strong> carcinoma by<br />
the administration of progestogen or progesterone.<br />
Unfortunately, this progestogenic opposition can lead<br />
to a resurgence of <strong>PMDD</strong>-like symptoms (not usually<br />
as severe as the original symptoms being treated). A<br />
previous study showed that <strong>PMDD</strong> could be recreated<br />
in postmenopausal women administered cyclical<br />
norethisterone. 11<br />
Attempts to avoid this (see Chapter 18) include<br />
reduction of the duration or dosage of progestogen, but<br />
this must be counterbalanced by the small increased<br />
risk of hyperplasia with progestogen restriction. Sturdee<br />
<strong>and</strong> colleagues showed that 12 days of progestogen<br />
avoids hyperplasia altogether, but a shorter duration