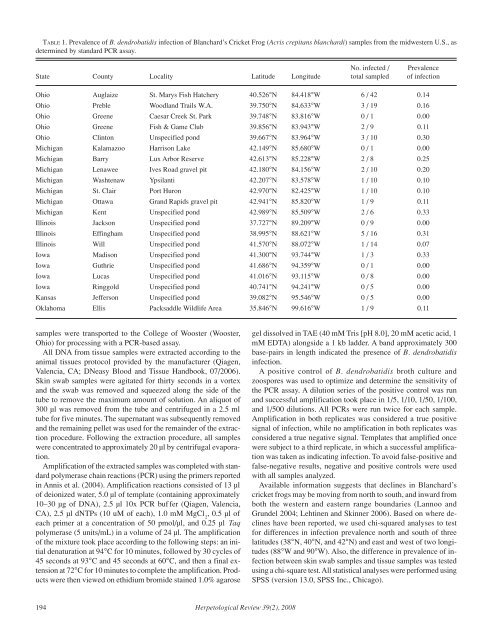
TABLE 1. Prevalence of B. dendrobatidis infection of Blanchard’s Cricket Frog (Acris crepitans blanchardi) samples from the midwestern U.S., asdetermined by standard PCR assay.No. infected / PrevalenceState County Locality Latitude Longitude total sampled of infectionOhio Auglaize St. Marys Fish Hatchery 40.526°N 84.418°W 6 / 42 0.14Ohio Preble Woodland Trails W.A. 39.750°N 84.633°W 3 / 19 0.16Ohio Greene Caesar Creek St. Park 39.748°N 83.816°W 0 / 1 0.00Ohio Greene Fish & Game Club 39.856°N 83.943°W 2 / 9 0.11Ohio Clinton Unspecified pond 39.667°N 83.964°W 3 / 10 0.30Michigan Kalamazoo Harrison Lake 42.149°N 85.680°W 0 / 1 0.00Michigan Barry Lux Arbor Reserve 42.613°N 85.228°W 2 / 8 0.25Michigan Lenawee Ives Road gravel pit 42.180°N 84.156°W 2 / 10 0.20Michigan Washtenaw Ypsilanti 42.207°N 83.578°W 1 / 10 0.10Michigan St. Clair Port Huron 42.970°N 82.425°W 1 / 10 0.10Michigan Ottawa Grand Rapids gravel pit 42.941°N 85.820°W 1 / 9 0.11Michigan Kent Unspecified pond 42.989°N 85.509°W 2 / 6 0.33Illinois Jackson Unspecified pond 37.727°N 89.209°W 0 / 9 0.00Illinois Effingham Unspecified pond 38.995°N 88.621°W 5 / 16 0.31Illinois Will Unspecified pond 41.570°N 88.072°W 1 / 14 0.07Iowa Madison Unspecified pond 41.300°N 93.744°W 1 / 3 0.33Iowa Guthrie Unspecified pond 41.686°N 94.359°W 0 / 1 0.00Iowa Lucas Unspecified pond 41.016°N 93.115°W 0 / 8 0.00Iowa Ringgold Unspecified pond 40.741°N 94.241°W 0 / 5 0.00Kansas Jefferson Unspecified pond 39.082°N 95.546°W 0 / 5 0.00Oklahoma Ellis Packsaddle Wildlife Area 35.846°N 99.616°W 1 / 9 0.11samples were transported to the College of Wooster (Wooster,Ohio) for processing with a PCR-based assay.All DNA from tissue samples were extracted according to theanimal tissues protocol provided by the manufacturer (Qiagen,Valencia, CA; DNeasy Blood and Tissue Handbook, 07/2006).Skin swab samples were agitated for thirty seconds in a vortexand the swab was removed and squeezed along the side of thetube to remove the maximum amount of solution. An aliquot of300 µl was removed from the tube and centrifuged in a 2.5 mltube for five minutes. The supernatant was subsequently removedand the remaining pellet was used for the remainder of the extractionprocedure. Following the extraction procedure, all sampleswere concentrated to approximately 20 µl by centrifugal evaporation.Amplification of the extracted samples was completed with standardpolymerase chain reactions (PCR) using the primers reportedin Annis et al. (2004). Amplification reactions consisted of 13 µlof deionized water, 5.0 µl of template (containing approximately10–30 µg of DNA), 2.5 µl 10x PCR buf fer (Qiagen, Valencia,CA), 2.5 µl dNTPs (10 uM of each), 1.0 mM MgCl 2, 0.5 µl ofeach primer at a concentration of 50 pmol/µl, and 0.25 µl Taqpolymerase (5 units/mL) in a volume of 24 µl. The amplificationof the mixture took place according to the following steps: an initialdenaturation at 94°C for 10 minutes, followed by 30 cycles of45 seconds at 93°C and 45 seconds at 60°C, and then a final extensionat 72°C for 10 minutes to complete the amplification. Productswere then viewed on ethidium bromide stained 1.0% agarosegel dissolved in TAE (40 mM Tris [pH 8.0], 20 mM acetic acid, 1mM EDTA) alongside a 1 kb ladder. A band approximately 300base-pairs in length indicated the presence of B. dendrobatidisinfection.A positive control of B. dendrobatidis broth culture andzoospores was used to optimize and determine the sensitivity ofthe PCR assay. A dilution series of the positive control was runand successful amplification took place in 1/5, 1/10, 1/50, 1/100,and 1/500 dilutions. All PCRs were run twice for each sample.Amplification in both replicates was considered a true positivesignal of infection, while no amplification in both replicates wasconsidered a true negative signal. Templates that amplified oncewere subject to a third replicate, in which a successful amplificationwas taken as indicating infection. To avoid false-positive andfalse-negative results, negative and positive controls were usedwith all samples analyzed.Available information suggests that declines in Blanchard’scricket frogs may be moving from north to south, and inward fromboth the western and eastern range boundaries (Lannoo andGrundel 2004; Lehtinen and Skinner 2006). Based on where declineshave been reported, we used chi-squared analyses to testfor differences in infection prevalence north and south of threelatitudes (38°N, 40°N, and 42°N) and east and west of two longitudes(88°W and 90°W). Also, the difference in prevalence of infectionbetween skin swab samples and tissue samples was testedusing a chi-square test. All statistical analyses were performed usingSPSS (version 13.0, SPSS Inc., Chicago).194 <strong>Herpetological</strong> <strong>Review</strong> 39(2), 2008
Results.—Batrachochytrium dendrobatidis was detected in15.1% (31 of 205) of samples from Blanchard’s Cricket Frogs(Table 1). Within infected sites, prevalence of infection was generallylow (18.8 ± 9.8%; Table 1). We did not detect B.dendrobatidis at seven sites but all of these localities had relativelysmall sample sizes. In all sites where more than ten frogswere sampled, B. dendrobatidis was detected (Table 1). No dead,dying or obviously diseased frogs were found at any of the investigatedsites.Infected frogs were found in Ohio, Michigan, Iowa, Illinois,and Oklahoma (but not in any of the five samples from Kansas;Table 1). There was no obvious geographic pattern in infection.Infection rates did not differ significantly at longitudes greaterand less than 90°W or 88°W (χ 2 = 1.281, df = 1, P = 0.258; χ 2 =1.130, df = 1, P = 0.288, respectively). Prevalence of infectionalso did not differ significantly at latitudes greater and less than38° N (χ 2 = 1.407, df = 1, P = 0.236), 40°N (χ 2 = 0.784, df = 1, P =0.376), or 42°N (χ 2 = 0.136, df = 1, P = 0.712). The prevalence ofinfection did not differ significantly between samples obtained byskin swabbing and those from tissue samples (χ 2 = .273, df = 1, P= .0602).Discussion.—We document the widespread presence of B.dendrobatidis in populations of Blanchard’s Cricket Frogs in themidwestern United States. Although B. dendrobatidis is nowknown from captive situations (Zippel and Tabaka 2008), to ourknowledge, this is the first documented case of B. dendrobatidisinfection in cricket frogs from wild populations. However, no mortalitywas found or reported in these infected, yet seemingly healthycricket frogs. Additionally, we have mark-recapture data from oneof these populations since 2004 (St. Marys Fish Hatchery, Ohio;R. Lehtinen, unpubl. data). This population has maintained a largeand relatively stable population size over the last four years (2004–2007), despite B. dendrobatidis infection. In this respect, our resultsparallel Ouellet et al. (2005) who found B. dendrobatidis tobe enzootic in Quebec with no symptoms associated with infection.It is possible that Blanchard’s Cricket Frogs utilize antimicrobialskin peptides (Woodhams et al. 2006) or behavioral thermoregulation(Woodhams et al. 2003) as defenses against B.dendrobatidis, but these possibilities have yet to be investigated.While preliminary, our data suggest that Blanchard’s Cricket Frogpopulations are able to persist in the face of ongoing B.dendrobatidis infection (e.g., Daszak et al. 2005; Retallick et al.2004).We found B. dendrobatidis to have no obvious geographic patternof occurrence. In fact, B. dendrobatidis was present at everysite where ten or more samples were available. These data reinforcethe observations of others that B. dendrobatidis appears tobe widespread in North America (Longcore et al. 2007; Ouellet etal. 2005; Pearl et al. 2007). Importantly, we detected B.dendrobatidis both in areas where Blanchard’s Cricket Frog declineshave occurred (Ohio, Michigan, northern Illinois) as wellas where declines have not been reported (Oklahoma, southernIllinois). The apparent absence of declines in more southerly partsof the range suggests that B. dendrobatidis may not be solely responsiblefor recent population declines in Blanchard’s CricketFrogs further north. However, more northerly populations couldbe more vulnerable to infection since B. dendrobatidis grows bestin cooler temperatures (Longcore et al. 1999) and the amphibianimmune system functions most effectively at higher temperatures(Maniero and Carey 1997). The declines in the northern portionsof the range could also have resulted from earlier declines causedby B. dendrobatidis that have now stabilized. Similar patterns ofpersistence with B. dendrobatidis after initial declines are known(Retallick et al. 2004; Woodhams and Alford 2005). More work isclearly needed to assess what threat (if any) B. dendrobatidis infectionposes to Blanchard’s Cricket Frogs.Many records of B. dendrobatidis infection in North Americananurans are from ranid and bufonid frogs (e.g., Ouellet et al. 2005;Pearl et al. 2007). Fewer hylid species have been reported to beinfected. Using histological techniques, Ouellet et al. (2005) reportedB. dendrobatidis infection in Pseudacris triseriata (54 outof 143 individuals) and Hyla versicolor (1 out of 16). However,Longcore et al. (2007) found no infection in Hyla versicolor (0out of 50), or Pseudacris crucifer (0 out of 21). Pearl et al. (2007)also found no infections in Pseudacris regilla (0 out of 28) in thePacific Northwest. Our results with Acris crepitans (31 out of 205)suggest that B. dendrobatidis may be more widely distributed inNorth American hylids than previously suspected.Acknowledgments.—We thank Dean Fraga for help with laboratoryprocedures and Nate Busman for help in the field. Janalee Caldwell, JeffDavis, Tony Gamble, Karen Kinkead, Chris Phillips, and Edi Sonntagcollected and sent us cricket frog skin swabs or tissue samples from theirrespective study areas and they have our sincere thanks for making theseavailable. We thank Corinne Richards for supplying the zoospores for thepositive control and the protocols for the skin swab DNA extraction procedure.We thank the Henry J. Copeland Fund and the Biology Departmentat the College of Wooster for funding this project which was a seniorIndependent Study project by the lead author. The Institutional AnimalCare and Use Committee at the College of Wooster approved themethods used in this project. Cricket frogs were captured in Ohio underpermit 06-266 from the Ohio Division of Wildlife and in Michigan undera scientific collector’s permit from the Michigan Department of NaturalResources.LITERATURE CITEDANNIS, S. L., F. P. DASTOOR, H. ZIEL, P. DASZAK, AND J. E. LONGCORE. 2004. ADNA-based assay identifies Batrachochytrium dendrobatidis in amphibians.J. Wildl. Diseases 40:420–428.BEASLEY, V. R., S. A. FAEH, B. WIKOFF, C. STAEHLE, J. EISOLD, D. NICHOLS,R. COLE, A. M. SCHOTTHOEFER, M. GREENWELL, AND L. E. BROWN. 2005.Risk factors and declines in northern cricket frogs (Acris crepitans). InM. J. Lannoo (ed.), Amphibian Declines: The Conservation Status ofUnited States Species, pp. 75–86. University of California Press, Berkeley.BERGER, L., SPEARE, R., DASZAK, P., GREEN, D. E., CUNNINGHAM, A. A.,GOGGIN, C. L., SLOCOMBE, R., RAGAN, M. A., HYATT, A. D., MCDONALD,K. R., HINES, H. B., LIPS, K. R., MARANTELLI, G., AND H. PARKES. 1998.Chytridiomycosis causes amphibian mortality associated with populationdeclines in the rain forests of Australia and Central America. PNAS95:9031–9036.BRODMAN, R., AND M. KILMURRY. 1998. Status of amphibians in northwesternIndiana. In M. J. Lannoo (ed.), Status and Conservation ofMidwestern Amphibians, pp. 125–136. University of Iowa Press, IowaCity.DASZAK, P., D. E. SCOTT, A. M. KILPATRICK, C. FAGGIONI, J. W. GIBBONS,AND D. PORTER. 2005. Amphibian population declines at Savannah Riversite are linked to climate, not chytridiomycosis. Ecology 86:3232–3237.GRAY, R. H., AND L. E. BROWN. 2005. Decline of northern cricket frogs(Acris crepitans). In M. J. Lannoo (ed.), Amphibian Declines: The<strong>Herpetological</strong> <strong>Review</strong> 39(2), 2008 195
- Page 1 and 2:
HerpetologicalReviewVolume 39, Numb
- Page 3 and 4:
About Our Cover: Zonosaurus maramai
- Page 5 and 6:
Prey-specific Predatory Behavior in
- Page 7 and 8:
acid water treatment than in the co
- Page 10 and 11:
TABLE 1. Time-line history of croco
- Page 12 and 13:
The Reptile House at the Bronx Zoo
- Page 14 and 15:
FIG. 6. A 3.9 m (12' 11 1 / 2") Ame
- Page 16 and 17:
One of the earliest studies of croc
- Page 18 and 19: TABLE 2. Dimensions and water depth
- Page 20 and 21: we call it, is in flux.Forty years
- Page 22 and 23: Feb. 20-25. abstract.------. 1979.
- Page 24 and 25: yond current practices (Clarke 1972
- Page 26 and 27: poles (Pond 1 > 10,000, Pond 2 4,87
- Page 28 and 29: ------, R. MATHEWS, AND R. KINGSING
- Page 30 and 31: Herpetological Review, 2008, 39(2),
- Page 32 and 33: TABLE 2. Summary of running (includ
- Page 34 and 35: FIG. 2. Responses of adult Regal Ho
- Page 36 and 37: PIANKA, E. R., AND W. S. PARKER. 19
- Page 38 and 39: BUSTAMANTE, M. R. 2005. La cecilia
- Page 40 and 41: Fig. 3. Mean clutch size (number of
- Page 42 and 43: facilitated work in Thailand. I tha
- Page 44 and 45: preocular are not fused. The specim
- Page 46 and 47: FIG. 2A) Side view photo of Aechmea
- Page 48 and 49: 364.DUELLMAN, W. E. 1978. The biolo
- Page 50 and 51: incision, and placed one drop of Ba
- Page 52 and 53: 13 cm deep (e.g., Spea hammondii; M
- Page 54 and 55: FIG. 1. Medicine dropper (60 ml) wi
- Page 56 and 57: esearchers and Hellbenders, especia
- Page 58 and 59: FIG. 3. Relative success of traps p
- Page 60 and 61: data on Hellbender population struc
- Page 62 and 63: aits sometimes resulted in differen
- Page 64 and 65: trapping system seems to be a relat
- Page 66 and 67: AMPHIBIAN CHYTRIDIOMYCOSISGEOGRAPHI
- Page 70 and 71: Conservation Status of United State
- Page 72 and 73: TABLE 1. Wood Frog (Rana sylvatica)
- Page 74 and 75: TABLE 1. Anurans that tested positi
- Page 76 and 77: is, on average, exposed to slightly
- Page 78 and 79: (10%) were dead but not obviously m
- Page 80 and 81: Submitted by CHRIS T. McALLISTER, D
- Page 82 and 83: FIG. 1. Oscillogram, spectrogram, a
- Page 84 and 85: FIG. 1. Adult Physalaemus cuvieri r
- Page 86 and 87: Répteis, Instituto Nacional de Pes
- Page 88 and 89: discovered 145 live hatchlings and
- Page 90 and 91: GRAPTEMYS GIBBONSI (Pascagoula Map
- Page 92 and 93: College, and the Joseph Moore Museu
- Page 94 and 95: FIG. 1. Common Ground Lizard (Ameiv
- Page 96 and 97: havior unavailable elsewhere. Here
- Page 98 and 99: 15% of predator mass, is typical fo
- Page 100 and 101: side the third burrow and began a f
- Page 102 and 103: We thank Arlington James and the st
- Page 104 and 105: mm) S. viridicornis in its mouth in
- Page 106 and 107: NECTURUS MACULOSUS (Common Mudpuppy
- Page 108 and 109: LITHOBATES CATESBEIANUS (American B
- Page 110 and 111: Research and Collections Center, 13
- Page 112 and 113: BRONCHOCELA VIETNAMENSIS (Vietnam L
- Page 114 and 115: Oficina Regional Guaymas, Guaymas,
- Page 116 and 117: MICRURUS TENER (Texas Coralsnake).
- Page 118 and 119:
declining in this recently discover
- Page 120 and 121:
80.7372°W). 02 November 2005. Stev
- Page 122 and 123:
this effort, 7% of the 10 × 10 km
- Page 124 and 125:
the knowledge of the group. The aut
- Page 126 and 127:
which is listed under “Rhodin, A.
- Page 128 and 129:
noting that Sphenomorphus bignelli
- Page 130 and 131:
256 Herpetological Review 39(2), 20
- Page 132:
ISSN 0018-084XThe Official News-Jou