Selectra Catheters - BIOTRONIK USA - News
Selectra Catheters - BIOTRONIK USA - News
Selectra Catheters - BIOTRONIK USA - News
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
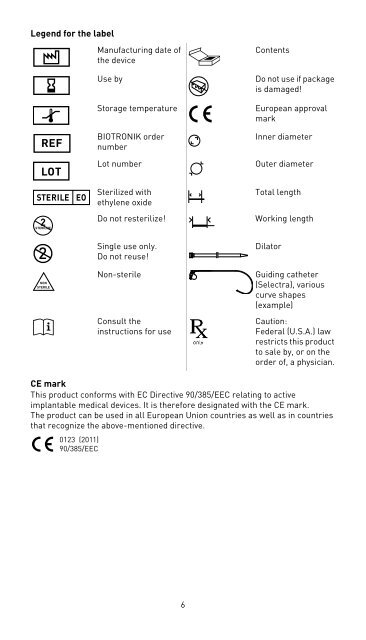
Legend for the labelManufacturing date ofthe deviceUse byStorage temperature<strong>BIOTRONIK</strong> ordernumberLot numberContentsDo not use if packageis damaged!European approvalmarkInner diameterOuter diameter2STERILIZESterilized withethylene oxideDo not resterilize!Total lengthWorking lengthNONSTERILESingle use only. Do not reuse!Non-sterileConsult theinstructions for useDilatorGuiding catheter(<strong>Selectra</strong>), variouscurve shapes(example)Caution:Federal (U.S.A.) lawrestricts this productto sale by, or on theorder of, a physician.CE markThis product conforms with EC Directive 90/385/EEC relating to activeimplantable medical devices. It is therefore designated with the CE mark. The product can be used in all European Union countries as well as in countriesthat recognize the above-mentioned directive.0123 (2011)90/385/EEC6