Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
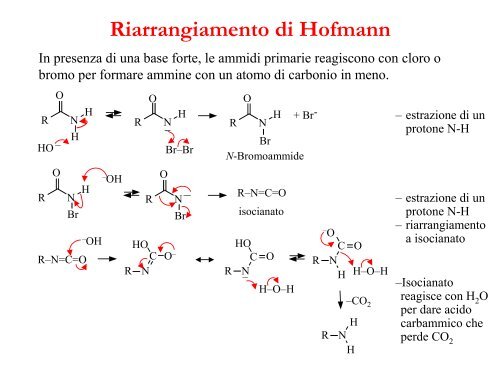
In presenza <strong>di</strong> una base forte, le ammi<strong>di</strong> primarie reagiscono con cloro obromo per formare ammine con un atomo <strong>di</strong> carbonio in meno.RHO –ROON HHN HBrR–N=C=O– OHRiarrangiamento <strong>di</strong> Hofmann– OHRORN H–OHOC O –R NBr–BrOR N HBr+ Br -N-BromoammideN– R–N=C=OBr isocianato– OHOC OC O R NR N – H H–O–HH–O–H–CO 2RHNH– estrazione <strong>di</strong> unprotone N-H– estrazione <strong>di</strong> unprotone N-H– riarrangiamentoa isocianato–Isocianatoreagisce con H 2Oper dare acidocarbammico cheperde CO 2