Årsrapport 2011 - Region Sjælland
Årsrapport 2011 - Region Sjælland
Årsrapport 2011 - Region Sjælland
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The SLAMSHAM study<br />
A Randomized Placebo Controlled Study of Arthroscopic Partial Meniscectomy<br />
Kristoffer B Hare, L Stefan Lohmander, Ewa M Roos<br />
PURPOSE<br />
The overall purpose of this study is to test whether the benefit from<br />
arthroscopic partial meniscectomy in patients aged 35-55 with knee<br />
pain and a degenerative meniscus lesion, is due to surgery or the<br />
placebo effect. Demonstration of no additional benefit from this<br />
surgery may reduce the development of surgically-related<br />
osteoarthritis in middle-aged patients.<br />
BACKGROUND<br />
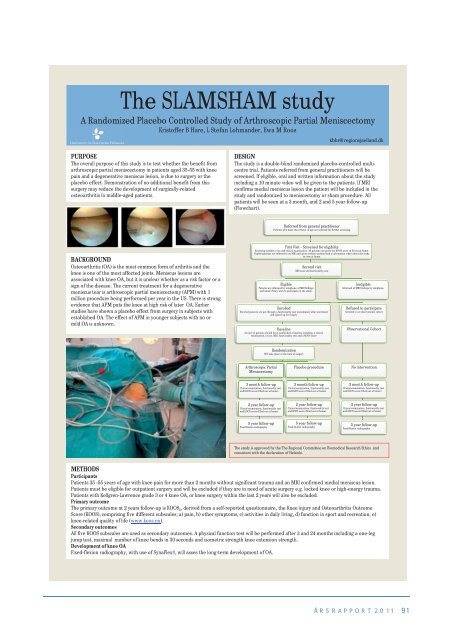
Osteoarthritis (OA) is the most common form of arthritis and the<br />
knee is one of the most affected joints. Meniscus lesions are<br />
associated with knee OA, but it is unclear whether as a risk factor or a<br />
sign of the disease. The current treatment for a degenerative<br />
meniscus tear is arthroscopic partial meniscectomy (APM) with 1<br />
million procedure being performed per year in the US. There is strong<br />
evidence that APM puts the knee at high risk of later OA. Earlier<br />
studies have shown a placebo effect from surgery in subjects with<br />
established OA. The effect of APM in younger subjects with no or<br />
mild OA is unknown.<br />
kbhr@regionsjaelland.dk<br />
DESIGN<br />
The study is a double-blind randomized placebo-controlled multicentre<br />
trial. Patients referred from general practitioners will be<br />
screened. If eligible, oral and written information about the study<br />
including a 10 minute video will be given to the patients. If MRI<br />
confirms medial meniscus lesion the patient will be included in the<br />
study and randomized to meniscectomy or sham procedure. All<br />
patients will be seen at a 3 month, and 2 and 5 year follow-up<br />
(Flowchart).<br />
Referred from general practitioner<br />
Patients who meet the criteria of age are selected for further screening<br />
First Visit - Screened for eligibility<br />
Screening includes x-ray and clinical examination. All patients are given the KOOS score to fill out at home.<br />
Eligible patients are referred to an MRI and given written material and a information video about the study<br />
to view at home.<br />
Second visit<br />
MRI scan and functionality test<br />
Eligible<br />
Patients are informed by telephone of MRI findings<br />
and asked if they wish to participate in the study.<br />
Enrolled<br />
Enrolled patients are put through a functionality test immediately after enrolment<br />
and signed up for surgery<br />
Baseline<br />
At now all patients should have established a baseline including, a clinical<br />
examination, x-rays, MRI, functionality test and a KOOS score<br />
Arthroscopic Partial<br />
Meniscectomy<br />
3 month follow-up<br />
Clinical examination, functionality test<br />
and KOOS score (filled out at home)<br />
2 year follow-up<br />
Clinical examination, functionality test<br />
and KOOS score (filled out at home)<br />
5 year follow-up<br />
Fixed-flexion radiography<br />
Randomization<br />
Will take place at the time of surgery<br />
Placebo procedure<br />
3 month follow-up<br />
Clinical examination, functionality test<br />
and KOOS score (filled out at home)<br />
2 year follow-up<br />
Clinical examination, functionality test<br />
and KOOS score (filled out at home)<br />
5 year follow-up<br />
Fixed-flexion radiography<br />
Ineligible<br />
Informed of MRI findings by telephone.<br />
Refused to participate<br />
Enrolled in an observational cohort<br />
Observational Cohort<br />
No intervention<br />
3 month follow-up<br />
Clinical examination, functionality test<br />
and KOOS score (filled out at home)<br />
2 year follow-up<br />
Clinical examination, functionality test<br />
and KOOS score (filled out at home)<br />
5 year follow-up<br />
Fixed-flexion radiography<br />
The study is approved by the The <strong>Region</strong>al Committee on Biomedical Research Ethics and<br />
consistent with the declaration of Helsinki.<br />
METHODS<br />
Participants<br />
Patients 35 -55 years of age with knee pain for more than 2 months without significant trauma and an MRI confirmed medial meniscus lesion.<br />
Patients must be eligible for outpatient surgery and will be excluded if they are in need of acute surgery e.g. locked knee or high-energy trauma.<br />
Patients with Kellgren-Lawrence grade 3 or 4 knee OA, or knee surgery within the last 2 years will also be excluded.<br />
Primary outcome<br />
The primary outcome at 2 years follow-up is KOOS 5, derived from a self-reported questionnaire, the Knee injury and Osteoarthritis Outcome<br />
Score (KOOS), comprising five different subscales; a) pain, b) other symptoms, c) activities in daily living, d) function in sport and recreation, e)<br />
knee-related quality of life (www.koos.nu).<br />
Secondary outcomes<br />
All five KOOS subscales are used as secondary outcomes. A physical function test will be performed after 3 and 24 months including a one-leg<br />
jump test, maximal number of knee bends in 30 seconds and isometric strength knee extension strength.<br />
Development of knee OA<br />
Fixed-flexion radiography, with use of SynaFlex®, will asses the long-term development of OA.<br />
ÅRSRAPPORT <strong>2011</strong> 91