Fugacity: It is derived from Latin, expressed as fleetness or escaping ...
Fugacity: It is derived from Latin, expressed as fleetness or escaping ...
Fugacity: It is derived from Latin, expressed as fleetness or escaping ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Non ideal solution<br />
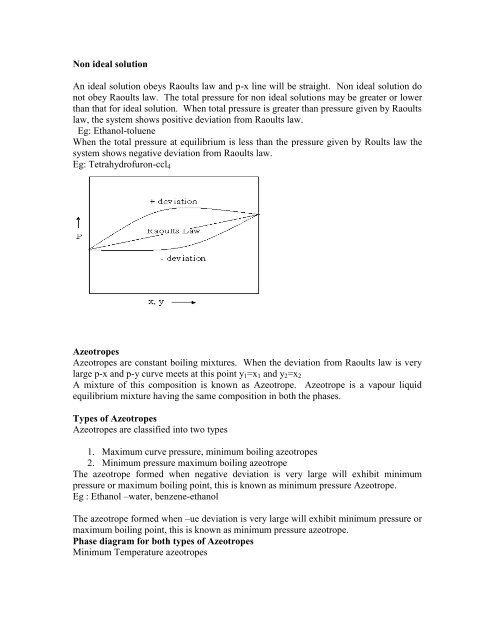
An ideal solution obeys Raoults law and p-x line will be straight. Non ideal solution do<br />
not obey Raoults law. The total pressure f<strong>or</strong> non ideal solutions may be greater <strong>or</strong> lower<br />
than that f<strong>or</strong> ideal solution. When total pressure <strong>is</strong> greater than pressure given by Raoults<br />
law, the system shows positive deviation <strong>from</strong> Raoults law.<br />
Eg: Ethanol-toluene<br />
When the total pressure at equilibrium <strong>is</strong> less than the pressure given by Roults law the<br />
system shows negative deviation <strong>from</strong> Raoults law.<br />
Eg: Tetrahydrofuron-ccl4<br />
Azeotropes<br />
Azeotropes are constant boiling mixtures. When the deviation <strong>from</strong> Raoults law <strong>is</strong> very<br />
large p-x and p-y curve meets at th<strong>is</strong> point y1=x1 and y2=x2<br />
A mixture of th<strong>is</strong> composition <strong>is</strong> known <strong>as</strong> Azeotrope. Azeotrope <strong>is</strong> a vapour liquid<br />
equilibrium mixture having the same composition in both the ph<strong>as</strong>es.<br />
Types of Azeotropes<br />
Azeotropes are cl<strong>as</strong>sified into two types<br />
1. Maximum curve pressure, minimum boiling azeotropes<br />
2. Minimum pressure maximum boiling azeotrope<br />
The azeotrope f<strong>or</strong>med when negative deviation <strong>is</strong> very large will exhibit minimum<br />
pressure <strong>or</strong> maximum boiling point, th<strong>is</strong> <strong>is</strong> known <strong>as</strong> minimum pressure Azeotrope.<br />
Eg : Ethanol –water, benzene-ethanol<br />
The azeotrope f<strong>or</strong>med when –ue deviation <strong>is</strong> very large will exhibit minimum pressure <strong>or</strong><br />
maximum boiling point, th<strong>is</strong> <strong>is</strong> known <strong>as</strong> minimum pressure azeotrope.<br />
Ph<strong>as</strong>e diagram f<strong>or</strong> both types of Azeotropes<br />
Minimum Temperature azeotropes