Vital Statistics Commission of Jamaica - Planning Institute of Jamaica
Vital Statistics Commission of Jamaica - Planning Institute of Jamaica
Vital Statistics Commission of Jamaica - Planning Institute of Jamaica
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
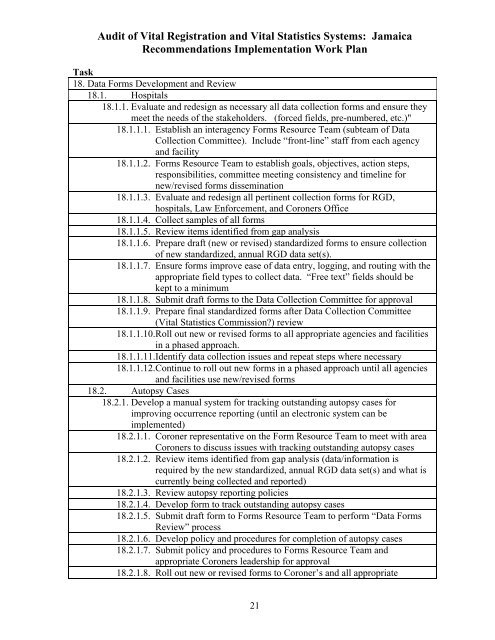
Audit <strong>of</strong> <strong>Vital</strong> Registration and <strong>Vital</strong> <strong>Statistics</strong> Systems: <strong>Jamaica</strong><br />
Recommendations Implementation Work Plan<br />
Task<br />
18. Data Forms Development and Review<br />
18.1. Hospitals<br />
18.1.1. Evaluate and redesign as necessary all data collection forms and ensure they<br />
meet the needs <strong>of</strong> the stakeholders. (forced fields, pre-numbered, etc.)"<br />
18.1.1.1. Establish an interagency Forms Resource Team (subteam <strong>of</strong> Data<br />
Collection Committee). Include “front-line” staff from each agency<br />
and facility<br />
18.1.1.2. Forms Resource Team to establish goals, objectives, action steps,<br />
responsibilities, committee meeting consistency and timeline for<br />
new/revised forms dissemination<br />
18.1.1.3. Evaluate and redesign all pertinent collection forms for RGD,<br />
hospitals, Law Enforcement, and Coroners Office<br />
18.1.1.4. Collect samples <strong>of</strong> all forms<br />
18.1.1.5. Review items identified from gap analysis<br />
18.1.1.6. Prepare draft (new or revised) standardized forms to ensure collection<br />
<strong>of</strong> new standardized, annual RGD data set(s).<br />
18.1.1.7. Ensure forms improve ease <strong>of</strong> data entry, logging, and routing with the<br />
appropriate field types to collect data. “Free text” fields should be<br />
kept to a minimum<br />
18.1.1.8. Submit draft forms to the Data Collection Committee for approval<br />
18.1.1.9. Prepare final standardized forms after Data Collection Committee<br />
(<strong>Vital</strong> <strong>Statistics</strong> <strong>Commission</strong>?) review<br />
18.1.1.10.Roll out new or revised forms to all appropriate agencies and facilities<br />
in a phased approach.<br />
18.1.1.11.Identify data collection issues and repeat steps where necessary<br />
18.1.1.12.Continue to roll out new forms in a phased approach until all agencies<br />
and facilities use new/revised forms<br />
18.2. Autopsy Cases<br />
18.2.1. Develop a manual system for tracking outstanding autopsy cases for<br />
improving occurrence reporting (until an electronic system can be<br />
implemented)<br />
18.2.1.1. Coroner representative on the Form Resource Team to meet with area<br />
Coroners to discuss issues with tracking outstanding autopsy cases<br />
18.2.1.2. Review items identified from gap analysis (data/information is<br />
required by the new standardized, annual RGD data set(s) and what is<br />
currently being collected and reported)<br />
18.2.1.3. Review autopsy reporting policies<br />
18.2.1.4. Develop form to track outstanding autopsy cases<br />
18.2.1.5. Submit draft form to Forms Resource Team to perform “Data Forms<br />
Review” process<br />
18.2.1.6. Develop policy and procedures for completion <strong>of</strong> autopsy cases<br />
18.2.1.7. Submit policy and procedures to Forms Resource Team and<br />
appropriate Coroners leadership for approval<br />
18.2.1.8. Roll out new or revised forms to Coroner’s and all appropriate<br />
21