Chitosan Loaded Mucoadhesive Microspheres of Gliclazide - Journal
Chitosan Loaded Mucoadhesive Microspheres of Gliclazide - Journal
Chitosan Loaded Mucoadhesive Microspheres of Gliclazide - Journal
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Prakash Rao B et al./ Formulation and Evaluation <strong>of</strong> <strong>Mucoadhesive</strong> Buccal Drug Delivery System <strong>of</strong> Metoprolol Tartrate by Using Central Composite Design<br />
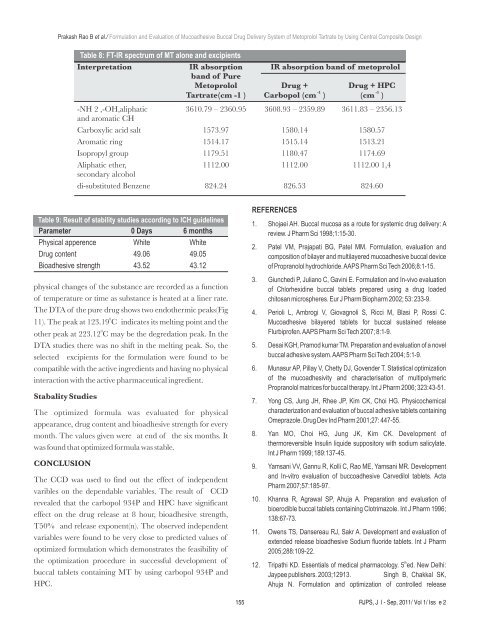
Table 8: FT-IR spectrum <strong>of</strong> MT alone and excipients<br />
Interpretation IR absorption IR absorption band <strong>of</strong> metoprolol<br />
band <strong>of</strong> Pure<br />
Metoprolol Drug + Drug + HPC<br />
Tartrate(cm -1 )<br />
-1<br />
Carbopol (cm )<br />
-1<br />
(cm )<br />
-NH 2 ,-OH,aliphatic 3610.79 – 2360.95 3608.93 – 2359.89 3611.83 – 2356.13<br />
and aromatic CH<br />
Carboxylic acid salt 1573.97 1580.14 1580.57<br />
Aromatic ring 1514.17 1515.14 1513.21<br />
Isopropyl group 1179.51 1180.47 1174.69<br />
Aliphatic ether, 1112.00 1112.00 1112.00 1,4<br />
secondary alcohol<br />
di-substituted Benzene 824.24 826.53 824.60<br />
Table 9: Result <strong>of</strong> stability studies according to ICH guidelines<br />
Parameter 0 Days 6 months<br />
Physical apperence White White<br />
Drug content 49.06 49.05<br />
Bioadhesive strength 43.52 43.12<br />
physical changes <strong>of</strong> the substance are recorded as a function<br />
<strong>of</strong> temperature or time as substance is heated at a liner rate.<br />
The DTA <strong>of</strong> the pure drug shows two endothermic peaks(Fig<br />
0<br />
11). The peak at 123.19 C indicates its melting point and the<br />
0<br />
other peak at 223.12 C may be the degredation peak. In the<br />
DTA studies there was no shift in the melting peak. So, the<br />
selected excipients for the formulation were found to be<br />
compatible with the active ingredients and having no physical<br />
interaction with the active pharmaceutical ingredient.<br />
Stabality Studies<br />
The optimized formula was evaluated for physical<br />
appearance, drug content and bioadhesive strength for every<br />
month. The values given were at end <strong>of</strong> the six months. It<br />
was found that optimized formula was stable.<br />
CONCLUSION<br />
The CCD was used to find out the effect <strong>of</strong> independent<br />
varibles on the dependable variables. The result <strong>of</strong> CCD<br />
revealed that the carbopol 934P and HPC have significant<br />
effect on the drug release at 8 hour, bioadhesive strength,<br />
T50% and release exponent(n). The observed independent<br />
variables were found to be very close to predicted values <strong>of</strong><br />
optimized formulation which demonstrates the feasibility <strong>of</strong><br />
the optimization procedure in successful development <strong>of</strong><br />
buccal tablets containing MT by using carbopol 934P and<br />
HPC.<br />
155<br />
REFERENCES<br />
1. Shojaei AH. Buccal mucosa as a route for systemic drug delivery: A<br />
review. J Pharm Sci 1998;1:15-30.<br />
2. Patel VM, Prajapati BG, Patel MM. Formulation, evaluation and<br />
composition <strong>of</strong> bilayer and multilayered mucoadhesive buccal device<br />
<strong>of</strong> Propranolol hydrochloride. AAPS Pharm Sci Tech 2006;8:1-15.<br />
3. Giunchedi P, Juliano C, Gavini E. Formulation and In-vivo evaluation<br />
<strong>of</strong> Chlorhexidine buccal tablets prepared using a drug loaded<br />
chitosan microspheres. Eur J Pharm Biopharm 2002; 53: 233-9.<br />
4. Perioli L, Ambrogi V, Giovagnoli S, Ricci M, Blasi P, Rossi C.<br />
<strong>Mucoadhesive</strong> bilayered tablets for buccal sustained release<br />
Flurbipr<strong>of</strong>en. AAPS Pharm Sci Tech 2007; 8:1-9.<br />
5. Desai KGH, Pramod kumar TM. Preparation and evaluation <strong>of</strong> a novel<br />
buccal adhesive system. AAPS Pharm Sci Tech 2004; 5:1-9.<br />
6. Munasur AP, Pillay V, Chetty DJ, Govender T. Statistical optimization<br />
<strong>of</strong> the mucoadhesivity and characterisation <strong>of</strong> multipolymeric<br />
Propranolol matrices for buccal therapy. Int J Pharm 2006; 323:43-51.<br />
7. Yong CS, Jung JH, Rhee JP, Kim CK, Choi HG. Physicochemical<br />
characterization and evaluation <strong>of</strong> buccal adhesive tablets containing<br />
Omeprazole. Drug Dev Ind Pharm 2001;27: 447-55.<br />
8. Yan MO, Choi HG, Jung JK, Kim CK. Development <strong>of</strong><br />
thermoreversible Insulin liquide suppository with sodium salicylate.<br />
Int J Pharm 1999; 189:137-45.<br />
9. Yamsani VV, Gannu R, Kolli C, Rao ME, Yamsani MR. Development<br />
and In-vitro evaluation <strong>of</strong> buccoadhesive Carvedilol tablets. Acta<br />
Pharm 2007;57:185-97.<br />
10. Khanna R, Agrawal SP, Ahuja A. Preparation and evaluation <strong>of</strong><br />
bioerodible buccal tablets containing Clotrimazole. Int J Pharm 1996;<br />
138:67-73.<br />
11. Owens TS, Dansereau RJ, Sakr A. Development and evaluation <strong>of</strong><br />
extended release bioadhesive Sodium fluoride tablets. Int J Pharm<br />
2005;288:109-22.<br />
12.<br />
th<br />
Tripathi KD. Essentials <strong>of</strong> medical pharmacology. 5 ed. New Delhi:<br />
Jaypee publishers. 2003;12913. Singh B, Chakkal SK,<br />
Ahuja N. Formulation and optimization <strong>of</strong> controlled release<br />
RJPS, Jul - Sep, 2011/ Vol 1/ Issue 2