Prevention Guide - Safe Handling of Hazardous Drugs - Irsst
Prevention Guide - Safe Handling of Hazardous Drugs - Irsst
Prevention Guide - Safe Handling of Hazardous Drugs - Irsst
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2. RISKS RELATED TO THE USE OF HAZARDOUS DRUGS<br />
2 RISKS RELATED TO THE USE OF HAZARDOUS DRUGS<br />
2.1 <strong>Hazardous</strong> <strong>Drugs</strong><br />
2.1.1 Definition <strong>of</strong> a Drug<br />
In Quebec, a drug is defined as any substance or mixture <strong>of</strong> substances that can be used:<br />
‣ for the diagnosis, treatment, alleviation or prevention <strong>of</strong> an illness, disorder, abnormal physical or<br />
psychological condition, or their symptoms, in people or animals; or<br />
‣ to restore, correct or modify the physiological functions <strong>of</strong> people or animals.<br />
There are two widely-used international classifications <strong>of</strong> drugs. The World Health Organization suggests the use<br />
<strong>of</strong> the anatomical, therapeutic and chemical (ATC) classification, while the American Hospital Formulary Service<br />
(AHFS) has a similar classification system. The Régie de l’assurance-maladie du Québec (Quebec Health<br />
Insurance Board) uses the AHFS classification as the Quebec frame <strong>of</strong> reference. The list <strong>of</strong> hazardous drugs<br />
proposed by NIOSH (Appendix 2) also uses this classification.<br />
This therapeutic classification groups the drugs according to their primary pharmacological effects (e.g., class<br />
10:00 – antineoplastic agents, class 68:00 - hormones and substitutes, etc.).<br />
For the purposes <strong>of</strong> this guide, antineoplastic drug includes such terms as anticancer drug and chemotherapy, as<br />
well as the drugs classified as such in the AHFS classification.<br />
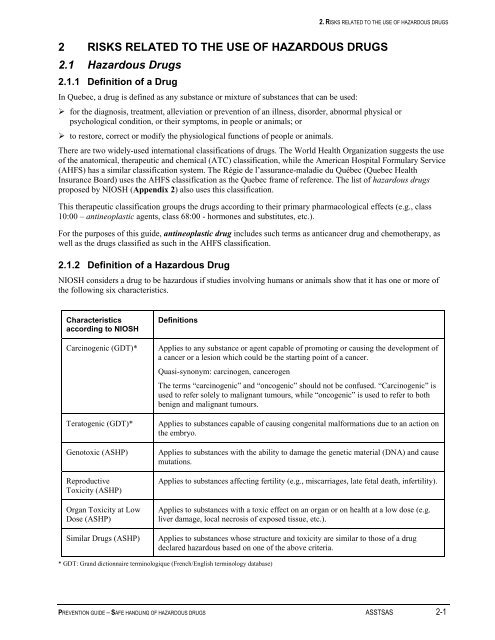
2.1.2 Definition <strong>of</strong> a <strong>Hazardous</strong> Drug<br />
NIOSH considers a drug to be hazardous if studies involving humans or animals show that it has one or more <strong>of</strong><br />
the following six characteristics.<br />
Characteristics<br />
according to NIOSH<br />
Carcinogenic (GDT)*<br />
Teratogenic (GDT)*<br />
Genotoxic (ASHP)<br />
Reproductive<br />
Toxicity (ASHP)<br />
Organ Toxicity at Low<br />
Dose (ASHP)<br />
Similar <strong>Drugs</strong> (ASHP)<br />
Definitions<br />
Applies to any substance or agent capable <strong>of</strong> promoting or causing the development <strong>of</strong><br />
a cancer or a lesion which could be the starting point <strong>of</strong> a cancer.<br />
Quasi-synonym: carcinogen, cancerogen<br />
The terms “carcinogenic” and “oncogenic” should not be confused. “Carcinogenic” is<br />
used to refer solely to malignant tumours, while “oncogenic” is used to refer to both<br />
benign and malignant tumours.<br />
Applies to substances capable <strong>of</strong> causing congenital malformations due to an action on<br />
the embryo.<br />
Applies to substances with the ability to damage the genetic material (DNA) and cause<br />
mutations.<br />
Applies to substances affecting fertility (e.g., miscarriages, late fetal death, infertility).<br />
Applies to substances with a toxic effect on an organ or on health at a low dose (e.g.<br />
liver damage, local necrosis <strong>of</strong> exposed tissue, etc.).<br />
Applies to substances whose structure and toxicity are similar to those <strong>of</strong> a drug<br />
declared hazardous based on one <strong>of</strong> the above criteria.<br />
* GDT: Grand dictionnaire terminologique (French/English terminology database)<br />
PREVENTION GUIDE – SAFE HANDLING OF HAZARDOUS DRUGS ASSTSAS 2-1