CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
molecular orbitals <strong>of</strong> thiophene oligomers and<br />
polymers form two continuous energy bands as<br />
opposed to discrete energy levels. 1 The energy<br />
gap (band gap) between the filled and vacant<br />
bands is approximately 4eV (~100 kcals), 1 which<br />
lies in the semiconductor regime. The 4eV band<br />
gap <strong>of</strong> thiophene oligomers and polymers, in<br />
conjunction with their stability and structural<br />
planarity, 1 allows quinquethiophene and other<br />
higher-order oligomers to mimic conventional<br />
semiconductors, such as silicon. In complement,<br />
the use <strong>of</strong> BNN radicals for the radical<br />
constituent <strong>of</strong> (1) was primarily motivated by the<br />
stability <strong>of</strong> these radicals 2 and their ability to be<br />
incorporated into a polymer backbone.<br />
Furthermore, the unpaired electron <strong>of</strong> the BNN<br />
radical is delocalized onto the annelated phenyl<br />
moiety in addition to the two NO groups, 2 thus<br />
providing multiple pathways for magnetic<br />
coupling between the radicals in three<br />
dimensions, in the solid state that can potentially<br />
translate into bulk ferromagnetic ordering <strong>of</strong><br />
electron spins 3 (which describes a magnetic<br />
material). Hence, a polythiophene polymer<br />
covalently “doped” with BNN radicals is<br />
inherently multifunctional and can, therefore,<br />
serve as a model system for investigating spincorrelated<br />
conductivity.<br />
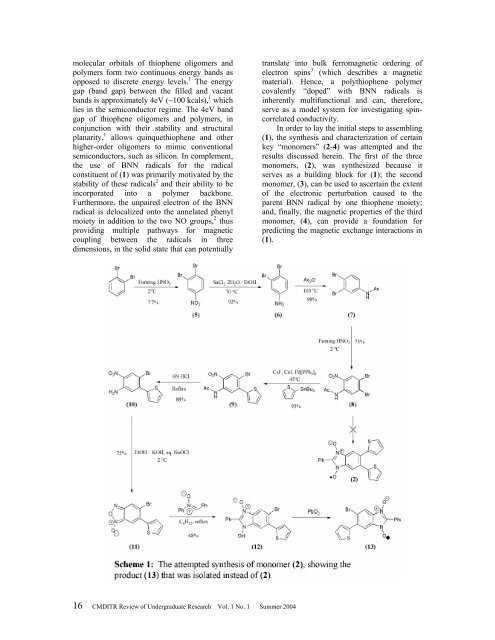
In order to lay the initial steps to assembling<br />
(1), the synthesis and characterization <strong>of</strong> certain<br />
key “monomers” (2-4) was attempted and the<br />
results discussed herein. The first <strong>of</strong> the three<br />
monomers, (2), was synthesized because it<br />
serves as a building block for (1); the second<br />
monomer, (3), can be used to ascertain the extent<br />
<strong>of</strong> the electronic perturbation caused to the<br />
parent BNN radical by one thiophene moiety;<br />
and, finally, the magnetic properties <strong>of</strong> the third<br />
monomer, (4), can provide a foundation for<br />
predicting the magnetic exchange interactions in<br />
(1).<br />
16 <strong>CMDITR</strong> <strong>Review</strong> <strong>of</strong> <strong>Undergraduate</strong> <strong>Research</strong> Vol. 1 No. 1 Summer 2004