CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
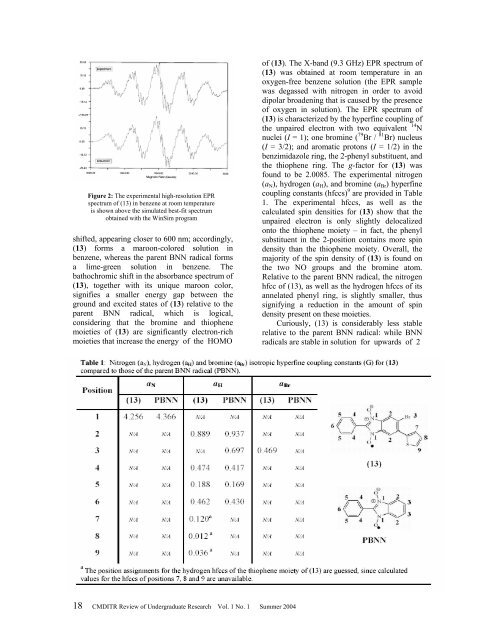
Figure 2: The experimental high-resolution EPR<br />
spectrum <strong>of</strong> (13) in benzene at room temperature<br />
is shown above the simulated best-fit spectrum<br />
obtained with the WinSim program<br />
shifted, appearing closer to 600 nm; accordingly,<br />
(13) forms a maroon-colored solution in<br />
benzene, whereas the parent BNN radical forms<br />
a lime-green solution in benzene. The<br />
bathochromic shift in the absorbance spectrum <strong>of</strong><br />
(13), together with its unique maroon color,<br />
signifies a smaller energy gap between the<br />
ground and excited states <strong>of</strong> (13) relative to the<br />
parent BNN radical, which is logical,<br />
considering that the bromine and thiophene<br />
moieties <strong>of</strong> (13) are significantly electron-rich<br />
moieties that increase the energy <strong>of</strong> the HOMO<br />
<strong>of</strong> (13). The X-band (9.3 GHz) EPR spectrum <strong>of</strong><br />
(13) was obtained at room temperature in an<br />
oxygen-free benzene solution (the EPR sample<br />
was degassed with nitrogen in order to avoid<br />
dipolar broadening that is caused by the presence<br />
<strong>of</strong> oxygen in solution). The EPR spectrum <strong>of</strong><br />
(13) is characterized by the hyperfine coupling <strong>of</strong><br />
the unpaired electron with two equivalent 14 N<br />
nuclei (I = 1); one bromine ( 79 Br / 81 Br) nucleus<br />
(I = 3/2); and aromatic protons (I = 1/2) in the<br />
benzimidazole ring, the 2-phenyl substituent, and<br />
the thiophene ring. The g-factor for (13) was<br />
found to be 2.0085. The experimental nitrogen<br />
(a N ), hydrogen (a H ), and bromine (a Br ) hyperfine<br />
coupling constants (hfccs) 9 are provided in Table<br />
1. The experimental hfccs, as well as the<br />
calculated spin densities for (13) show that the<br />
unpaired electron is only slightly delocalized<br />
onto the thiophene moiety – in fact, the phenyl<br />
substituent in the 2-position contains more spin<br />
density than the thiophene moiety. Overall, the<br />
majority <strong>of</strong> the spin density <strong>of</strong> (13) is found on<br />
the two NO groups and the bromine atom.<br />
Relative to the parent BNN radical, the nitrogen<br />
hfcc <strong>of</strong> (13), as well as the hydrogen hfccs <strong>of</strong> its<br />
annelated phenyl ring, is slightly smaller, thus<br />
signifying a reduction in the amount <strong>of</strong> spin<br />
density present on these moieties.<br />
Curiously, (13) is considerably less stable<br />
relative to the parent BNN radical: while BNN<br />
radicals are stable in solution for upwards <strong>of</strong> 2<br />
18 <strong>CMDITR</strong> <strong>Review</strong> <strong>of</strong> <strong>Undergraduate</strong> <strong>Research</strong> Vol. 1 No. 1 Summer 2004