CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
years, (13) decomposes into a yellow oil (in<br />
the absence <strong>of</strong> oxygen) after two days.<br />
Moreover, the presence <strong>of</strong> water can further<br />
reduce the lifetime <strong>of</strong> (13) to approximately<br />
twenty-two hours. From concurrent work in<br />
our lab 10 , it was discovered that, in general,<br />
a loss <strong>of</strong> stability in derivatives or analogs<br />
<strong>of</strong> BNN radicals is concomitant with a rise<br />
in electron deficiency; therefore, the<br />
instability <strong>of</strong> (13) relative to the parent BNN<br />
radical is in agreement with this general<br />
trend, since the bromine substituent on the<br />
benzimidazole ring renders (13) extremely<br />
electron deficient.<br />
Synthesis <strong>of</strong> (3)<br />
Since it was found earlier that (2) could<br />
not be synthesized via a Stille crosscoupling<br />
reaction due to steric hindrance,<br />
the synthesis <strong>of</strong> (3) was undertaken in an<br />
attempt to quantify the electronic<br />
perturbation caused to the parent BNN<br />
radical by a thiophene moiety in the absence<br />
<strong>of</strong> a bromine substituent.<br />
The synthesis <strong>of</strong> (3) closely resembles the<br />
synthesis <strong>of</strong> (2) – p-bromoaniline is N-protected<br />
and nitrated and the product cross-coupled with<br />
2-(tri-n-butylstannyl)-thiophene to yield 2-nitro-<br />
4-(2-thienyl)-acetanilide 6 (16), which is then<br />
deprotected and oxidized to the corresponding<br />
furoxan (18) under the same conditions as (9). 8<br />
The precursor to (3) is, once again, obtained by<br />
condensing (18) with phenyl nitrone in<br />
cyclohexane, albeit at a lower temperature than<br />
(11). (3) can then be obtained by the oxidation <strong>of</strong><br />
(19) with excess PbO 2 in benzene or<br />
dichloromethane.<br />
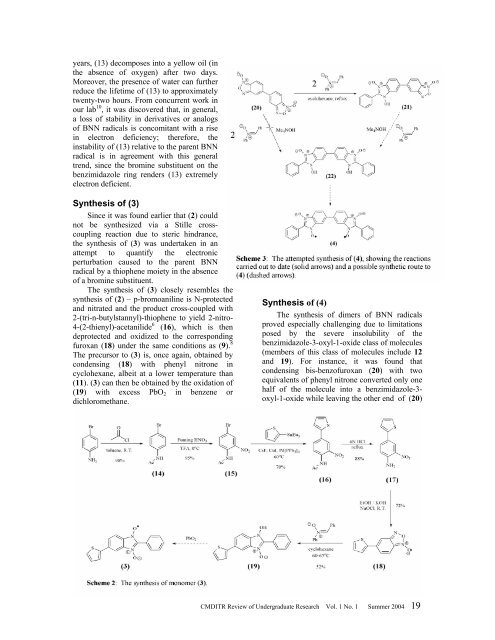
Synthesis <strong>of</strong> (4)<br />
The synthesis <strong>of</strong> dimers <strong>of</strong> BNN radicals<br />
proved especially challenging due to limitations<br />
posed by the severe insolubility <strong>of</strong> the<br />
benzimidazole-3-oxyl-1-oxide class <strong>of</strong> molecules<br />
(members <strong>of</strong> this class <strong>of</strong> molecules include 12<br />
and 19). For instance, it was found that<br />
condensing bis-benz<strong>of</strong>uroxan (20) with two<br />
equivalents <strong>of</strong> phenyl nitrone converted only one<br />
half <strong>of</strong> the molecule into a benzimidazole-3-<br />
oxyl-1-oxide while leaving the other end <strong>of</strong> (20)<br />
<strong>CMDITR</strong> <strong>Review</strong> <strong>of</strong> <strong>Undergraduate</strong> <strong>Research</strong> Vol. 1 No. 1 Summer 2004 19