CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
chromatography (TLC) (5%EA in methylene<br />
chloride) was performed on the irradiated and<br />
non-irradiated solutions. Irradiated solution<br />
showed separation into a light blue colored band<br />
(non-degraded) as well as pale-yellow substance<br />
(degraded FTC). With this data in hand, multiple<br />
samples <strong>of</strong> degraded chromophore were<br />
collected. Then, EA was dried out from those<br />
solutions leaving the solid that had a brownishorange<br />
color. NMR scan <strong>of</strong> that solid finally<br />
showed some new and different peaks in<br />
comparison to the NMR scan <strong>of</strong> the original,<br />
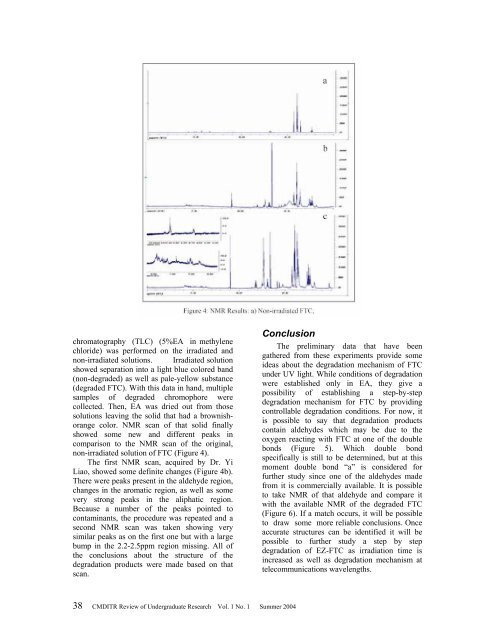
non-irradiated solution <strong>of</strong> FTC (Figure 4).<br />
The first NMR scan, acquired by Dr. Yi<br />
Liao, showed some definite changes (Figure 4b).<br />
There were peaks present in the aldehyde region,<br />
changes in the aromatic region, as well as some<br />
very strong peaks in the aliphatic region.<br />
Because a number <strong>of</strong> the peaks pointed to<br />
contaminants, the procedure was repeated and a<br />
second NMR scan was taken showing very<br />
similar peaks as on the first one but with a large<br />
bump in the 2.2-2.5ppm region missing. All <strong>of</strong><br />
the conclusions about the structure <strong>of</strong> the<br />
degradation products were made based on that<br />
scan.<br />
Conclusion<br />
The preliminary data that have been<br />
gathered from these experiments provide some<br />
ideas about the degradation mechanism <strong>of</strong> FTC<br />
under UV light. While conditions <strong>of</strong> degradation<br />
were established only in EA, they give a<br />
possibility <strong>of</strong> establishing a step-by-step<br />
degradation mechanism for FTC by providing<br />
controllable degradation conditions. For now, it<br />
is possible to say that degradation products<br />
contain aldehydes which may be due to the<br />
oxygen reacting with FTC at one <strong>of</strong> the double<br />
bonds (Figure 5). Which double bond<br />
specifically is still to be determined, but at this<br />
moment double bond “a” is considered for<br />
further study since one <strong>of</strong> the aldehydes made<br />
from it is commercially available. It is possible<br />
to take NMR <strong>of</strong> that aldehyde and compare it<br />
with the available NMR <strong>of</strong> the degraded FTC<br />
(Figure 6). If a match occurs, it will be possible<br />
to draw some more reliable conclusions. Once<br />
accurate structures can be identified it will be<br />
possible to further study a step by step<br />
degradation <strong>of</strong> EZ-FTC as irradiation time is<br />
increased as well as degradation mechanism at<br />
telecommunications wavelengths.<br />
38 <strong>CMDITR</strong> <strong>Review</strong> <strong>of</strong> <strong>Undergraduate</strong> <strong>Research</strong> Vol. 1 No. 1 Summer 2004