CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
CMDITR Review of Undergraduate Research - Pluto - University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
the source <strong>of</strong> the light emitted by the device.<br />
When the electron recombines with the hole, it is<br />
simply returning to its ground state. In doing so,<br />
it releases energy in the form <strong>of</strong> a photon. The<br />
higher the electron’s excited state, the shorter the<br />
wavelength <strong>of</strong> the photon it emits when it<br />
decays. This difference in energy between the<br />
excited state and the ground state is a material’s<br />
“bandgap”. The bandgap essentially determines<br />
the frequency <strong>of</strong> a photon that a material will<br />
emit. To create blue emitting materials, for<br />
example, the bandgap must be quite large,<br />
around 3.0eV.<br />
There are two particular ways in which an<br />
exciton decays. These processes are called<br />
fluorescence and phosphorescence, and they<br />
depend on the type <strong>of</strong> the exciton. The spins <strong>of</strong><br />
the ground state and excited state electrons<br />
determine its type. The two states are referred to<br />
as “singlet” and “triplet”. The relationship<br />
between the spins <strong>of</strong> the electron in the excited<br />
state and the electron in the ground state defines<br />
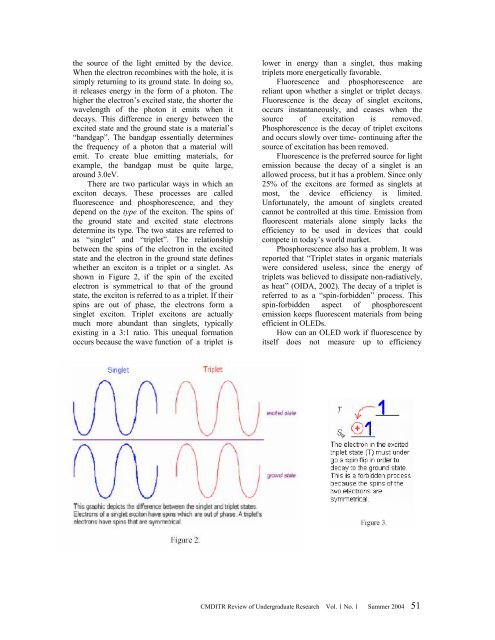
whether an exciton is a triplet or a singlet. As<br />
shown in Figure 2, if the spin <strong>of</strong> the excited<br />
electron is symmetrical to that <strong>of</strong> the ground<br />
state, the exciton is referred to as a triplet. If their<br />
spins are out <strong>of</strong> phase, the electrons form a<br />
singlet exciton. Triplet excitons are actually<br />
much more abundant than singlets, typically<br />
existing in a 3:1 ratio. This unequal formation<br />
occurs because the wave function <strong>of</strong> a triplet is<br />
lower in energy than a singlet, thus making<br />
triplets more energetically favorable.<br />
Fluorescence and phosphorescence are<br />
reliant upon whether a singlet or triplet decays.<br />
Fluorescence is the decay <strong>of</strong> singlet excitons,<br />
occurs instantaneously, and ceases when the<br />
source <strong>of</strong> excitation is removed.<br />
Phosphorescence is the decay <strong>of</strong> triplet excitons<br />
and occurs slowly over time- continuing after the<br />
source <strong>of</strong> excitation has been removed.<br />
Fluorescence is the preferred source for light<br />
emission because the decay <strong>of</strong> a singlet is an<br />
allowed process, but it has a problem. Since only<br />
25% <strong>of</strong> the excitons are formed as singlets at<br />
most, the device efficiency is limited.<br />
Unfortunately, the amount <strong>of</strong> singlets created<br />
cannot be controlled at this time. Emission from<br />
fluorescent materials alone simply lacks the<br />
efficiency to be used in devices that could<br />
compete in today’s world market.<br />
Phosphorescence also has a problem. It was<br />
reported that “Triplet states in organic materials<br />
were considered useless, since the energy <strong>of</strong><br />
triplets was believed to dissipate non-radiatively,<br />
as heat” (OIDA, 2002). The decay <strong>of</strong> a triplet is<br />
referred to as a “spin-forbidden” process. This<br />
spin-forbidden aspect <strong>of</strong> phosphorescent<br />
emission keeps fluorescent materials from being<br />
efficient in OLEDs.<br />
How can an OLED work if fluorescence by<br />
itself does not measure up to efficiency<br />
<strong>CMDITR</strong> <strong>Review</strong> <strong>of</strong> <strong>Undergraduate</strong> <strong>Research</strong> Vol. 1 No. 1 Summer 2004 51