Guide to the implementation of directives based on the New ...
Guide to the implementation of directives based on the New ...
Guide to the implementation of directives based on the New ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
❝ Annex 8 ❞<br />
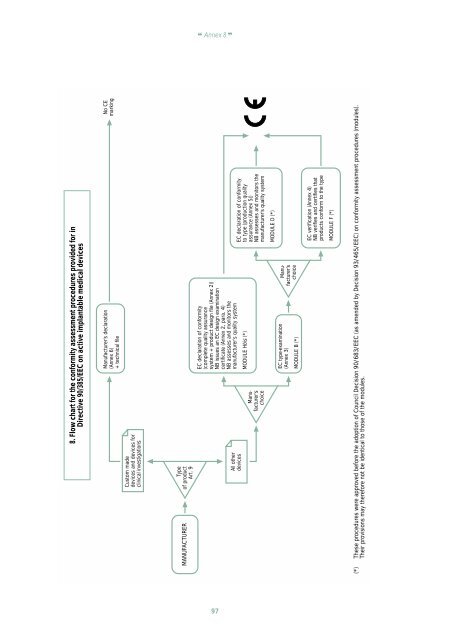
8. Flow chart for <str<strong>on</strong>g>the</str<strong>on</strong>g> c<strong>on</strong>formity assessment procedures provided for in<br />
Directive 90/385/EEC <strong>on</strong> active implantable medical devices<br />
Manufacturer’s declarati<strong>on</strong><br />
(Annex 6)<br />
+ technical file<br />
No CE<br />
marking<br />
Cus<str<strong>on</strong>g>to</str<strong>on</strong>g>m made<br />
devices and devices for<br />
clinical investigati<strong>on</strong>s<br />
MANUFACTURER<br />
Type<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> product<br />
Art. 9<br />
All o<str<strong>on</strong>g>the</str<strong>on</strong>g>r<br />
devices<br />
Manufacturer’s<br />
choice<br />
EC declarati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>formity<br />
(complete quality assurance<br />
system + product design file (Annex 2))<br />
NB issues an EC design examinati<strong>on</strong><br />
certificate (Annex 2, para. 4)<br />
NB assesses and m<strong>on</strong>i<str<strong>on</strong>g>to</str<strong>on</strong>g>rs <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
manufacturer’s quality system<br />
MODULE Hbis (*)<br />
EC declarati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>formity<br />
<str<strong>on</strong>g>to</str<strong>on</strong>g> type (producti<strong>on</strong> quality<br />
assurance (Annex 5))<br />
NB assesses and m<strong>on</strong>i<str<strong>on</strong>g>to</str<strong>on</strong>g>rs <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
manufacturer’s quality system<br />
EC type-examinati<strong>on</strong><br />
(Annex 3)<br />
MODULE B (*)<br />
Manufacturer’s<br />
choice<br />
MODULE D (*)<br />
EC verificati<strong>on</strong> (Annex 4)<br />
NB verifies and certifies that<br />
products c<strong>on</strong>form <str<strong>on</strong>g>to</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> type<br />
MODULE F (*)<br />
(*) These procedures were approved before <str<strong>on</strong>g>the</str<strong>on</strong>g> adopti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> Council Decisi<strong>on</strong> 90/683/EEC (as amended by Decisi<strong>on</strong> 93/465/EEC) <strong>on</strong> c<strong>on</strong>formity assessment procedures (modules).<br />
Their provisi<strong>on</strong>s may <str<strong>on</strong>g>the</str<strong>on</strong>g>refore not be identical <str<strong>on</strong>g>to</str<strong>on</strong>g> those <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> modules.<br />
97