Rad Data Handbook 20.. - Voss Associates
Rad Data Handbook 20.. - Voss Associates
Rad Data Handbook 20.. - Voss Associates
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
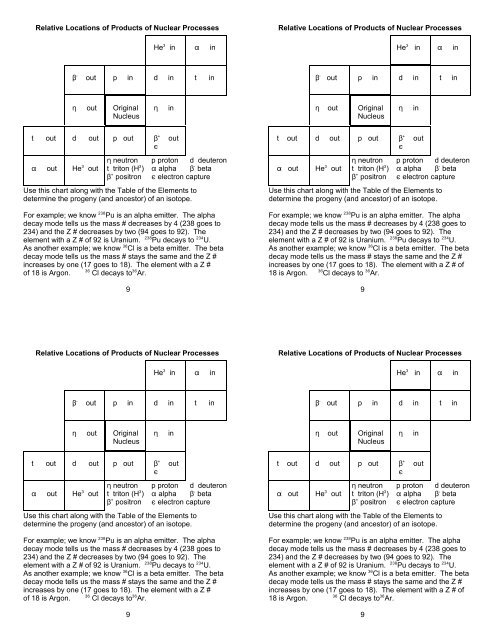
Relative Locations of Products of Nuclear Processes Relative Locations of Products of Nuclear Processes<br />
3<br />
He in in<br />
3<br />
He in in<br />
-<br />
out p in d in t in<br />
-<br />
out p in d in t in<br />
out Original<br />
Nucleus<br />
in out Original<br />
Nucleus<br />
<br />
in<br />
t out d out p out<br />
+<br />
out<br />
<br />
3<br />
out He out<br />
neutron p proton d deuteron<br />
3<br />
t triton (H ) alpha<br />
-<br />
beta<br />
+<br />
positron electron capture<br />
Use this chart along with the Table of the Elements to<br />
determine the progeny (and ancestor) of an isotope.<br />
238<br />
For example; we know Pu is an alpha emitter. The alpha<br />
decay mode tells us the mass # decreases by 4 (238 goes to<br />
234) and the Z # decreases by two (94 goes to 92). The<br />
238 234<br />
element with a Z # of 92 is Uranium. Pu decays to U.<br />
36<br />
As another example; we know Cl is a beta emitter. The beta<br />
decay mode tells us the mass # stays the same and the Z #<br />
increases by one (17 goes to 18). The element with a Z #<br />
36 36<br />
of 18 is Argon. Cl decays to Ar.<br />
9<br />
t out d out p out<br />
+<br />
out<br />
<br />
3<br />
out He out<br />
neutron p proton d deuteron<br />
3<br />
t triton (H ) alpha<br />
-<br />
beta<br />
+<br />
positron electron capture<br />
Use this chart along with the Table of the Elements to<br />
determine the progeny (and ancestor) of an isotope.<br />
238<br />
For example; we know Pu is an alpha emitter. The alpha<br />
decay mode tells us the mass # decreases by 4 (238 goes to<br />
234) and the Z # decreases by two (94 goes to 92). The<br />
238 234<br />
element with a Z # of 92 is Uranium. Pu decays to U.<br />
36<br />
As another example; we know Cl is a beta emitter. The beta<br />
decay mode tells us the mass # stays the same and the Z #<br />
increases by one (17 goes to 18). The element with a Z # of<br />
36 36<br />
18 is Argon. Cl decays to Ar.<br />
9<br />
Relative Locations of Products of Nuclear Processes Relative Locations of Products of Nuclear Processes<br />
3<br />
He in in<br />
3<br />
He in in<br />
-<br />
out p in d in t in<br />
-<br />
out p in d in t in<br />
out Original<br />
Nucleus<br />
in out Original<br />
Nucleus<br />
<br />
in<br />
t out d out p out<br />
+<br />
out<br />
<br />
3<br />
out He out<br />
neutron p proton d deuteron<br />
3<br />
t triton (H ) alpha<br />
-<br />
beta<br />
+<br />
positron electron capture<br />
Use this chart along with the Table of the Elements to<br />
determine the progeny (and ancestor) of an isotope.<br />
238<br />
For example; we know Pu is an alpha emitter. The alpha<br />
decay mode tells us the mass # decreases by 4 (238 goes to<br />
234) and the Z # decreases by two (94 goes to 92). The<br />
238 234<br />
element with a Z # of 92 is Uranium. Pu decays to U.<br />
36<br />
As another example; we know Cl is a beta emitter. The beta<br />
decay mode tells us the mass # stays the same and the Z #<br />
increases by one (17 goes to 18). The element with a Z #<br />
36 36<br />
of 18 is Argon. Cl decays to Ar.<br />
9<br />
t out d out p out<br />
+<br />
out<br />
<br />
3<br />
out He out<br />
neutron p proton d deuteron<br />
3<br />
t triton (H ) alpha<br />
-<br />
beta<br />
+<br />
positron electron capture<br />
Use this chart along with the Table of the Elements to<br />
determine the progeny (and ancestor) of an isotope.<br />
238<br />
For example; we know Pu is an alpha emitter. The alpha<br />
decay mode tells us the mass # decreases by 4 (238 goes to<br />
234) and the Z # decreases by two (94 goes to 92). The<br />
238 234<br />
element with a Z # of 92 is Uranium. Pu decays to U.<br />
36<br />
As another example; we know Cl is a beta emitter. The beta<br />
decay mode tells us the mass # stays the same and the Z #<br />
increases by one (17 goes to 18). The element with a Z # of<br />
36 36<br />
18 is Argon. Cl decays to Ar.<br />
9