- Page 1 and 2:
Silica Immobilised MetalIon Activat
- Page 3 and 4:
ABSTRACTImmobilisation of functiona
- Page 5:
DECLARATIONI certify that this thes
- Page 8 and 9:
EtOHGPDMSGPDMESGPSGPTSethanolglycid
- Page 10:
CHAPTER ONEINTRODUCTION
- Page 13 and 14:
The focus of this work was to prepa
- Page 15 and 16:
sequestering anions has proved to b
- Page 17 and 18:
ange of pH due to its high basicity
- Page 19 and 20:
nitrophenol or p-nitrophenolate wit
- Page 21 and 22:
It has been shown by NMR and crysta
- Page 23 and 24:
within the cavity of a p-tert-butyl
- Page 25 and 26:
60 in 0.5% DMSO-MeCN (v/v). 82 The
- Page 27 and 28:
synthesised bi- tri- and tetra - π
- Page 29 and 30:
adopt a trans-III conformation, whe
- Page 31 and 32:
amide oxygen atoms on the pendant a
- Page 33 and 34:
apparent there is, nonetheless, evi
- Page 35 and 36:
However, hetero-N-substitution on t
- Page 37 and 38:
Si-OH +- OH SiO - + H 2 OFor surfac
- Page 39 and 40:
35This material was obtained with a
- Page 41 and 42:
OOSiOHOROR OSiOHOOOSiOSiOSiOSiOSiOS
- Page 43 and 44:
Tetraaza metal complexes immobilise
- Page 45 and 46:
CHAPTER TWOSYNTHESIS OF MACROCYCLIC
- Page 47 and 48:
In addition, successful immobilisat
- Page 49 and 50:
3233Figure 2.3Reaction of 3-(glycid
- Page 51 and 52:
The methodology chosen was adapted
- Page 53 and 54:
agreed with literature values. 109R
- Page 55 and 56:
XS HO O H61, n = 262, n = 3n+ClOSO6
- Page 57 and 58:
anhydrous ethanol, Scheme 2.7. The
- Page 59 and 60:
75Figure 2.5 Structure of (S)-(-)-p
- Page 61 and 62:
metal ion coordination the pendant
- Page 63 and 64:
CHAPTER THREEGUEST MOLECULE INCLUSI
- Page 65 and 66:
3.1 where K A is the association, o
- Page 67 and 68:
0.5cχG∆δ∆δGbaχ GFigure 3.1S
- Page 69 and 70:
χG∆δ∆δGFigure 3.4 Job's Plot
- Page 71 and 72:
The downfield movement of the guest
- Page 73 and 74:
arm framework. The relative magnitu
- Page 75 and 76:
This phenomenon correlates well wit
- Page 77 and 78: non-classical hydrogen bonding is g
- Page 79 and 80: The full set of logK values obtaine
- Page 81 and 82: such that they would indicate precl
- Page 83 and 84: CHAPTER 4MODIFICATION OF THE SILICA
- Page 85 and 86: Chemical modification of the silica
- Page 87 and 88: GPTSGPDMESFigure 4.4Schematic depic
- Page 89 and 90: GPS, 33, linker material with an ex
- Page 91 and 92: free νOHτSiOHνSi-O-SiδH 2 Oasym
- Page 93 and 94: 224, 225the surface silanol groups.
- Page 95 and 96: esonances, the spectrum for Si-GPS-
- Page 97 and 98: increase in surface coverage from t
- Page 99 and 100: and, more so due to the larger size
- Page 101 and 102: CHAPTER 5METAL ION UPTAKE STUDIES W
- Page 103 and 104: Scheme 5.1 Metal(II) ion coordinati
- Page 105 and 106: linker material, Si-GPS, 33; endcap
- Page 107 and 108: [Cd(Trac)](ClO 4 ) 2 , 94, or Si-GP
- Page 109 and 110: CHAPTER 6GUEST MOLECULE INCLUSION S
- Page 111 and 112: and D-histidinate. Guest inclusion
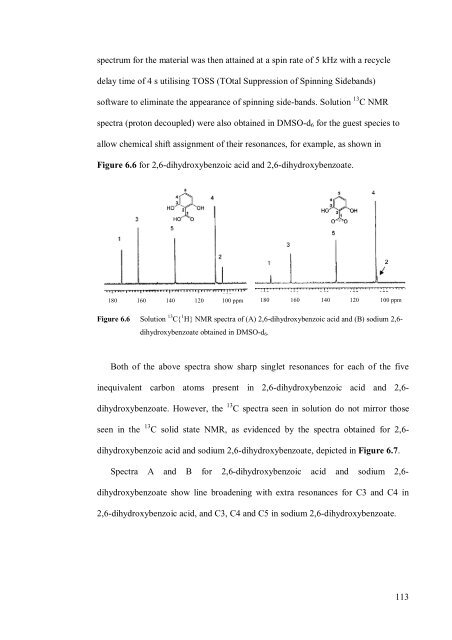
- Page 113 and 114: Table 6.2Percent inclusion data for
- Page 115 and 116: contains two hydroxy groups in the
- Page 117 and 118: methoxy group, as portrayed by the
- Page 119 and 120: these guest molecules with receptor
- Page 121 and 122: only about half of that taken up by
- Page 123 and 124: Percent inclusion values for these
- Page 125 and 126: Table 6.7Percent guest inclusion of
- Page 127: 6.1.7 Guest inclusion studies with
- Page 131 and 132: A1 46 7532 Anionic Guest8BCHostHost
- Page 133 and 134: extent, meta-positioned hydroxy gro
- Page 135 and 136: δ obs (ppm)6.2146.2136.2126.2116.2
- Page 137 and 138: CHAPTER 7EXPERIMENTAL7.1 General Ex
- Page 139 and 140: inding constants (logK values) and
- Page 141 and 142: delay time of 4 s. 13 C CPMAS NMR s
- Page 143 and 144: C, -CH 2 - CH 2 OSO 2 ); 68.11 (1 C
- Page 145 and 146: A solution of 69 (2.00 g, 10.1 mmol
- Page 147 and 148: Bn, meta); 127.79 (1 C, Bn, para);
- Page 149 and 150: added in a single portion. The mixt
- Page 151 and 152: dioxane): δ 158.67 (1 C, C=O); 135
- Page 153 and 154: 1-(benzyloxycarbonyl)-4,7,10-tris-(
- Page 155 and 156: 1,4,7-tris-((S)-2-hydroxy-3-phenoxy
- Page 157 and 158: 1,4,7-tris(S)-2-hydroxy-3-[4-((2-(2
- Page 159 and 160: 1,4,7-tris(S)-2-hydroxy-3-[4-(2-(2-
- Page 161 and 162: 1-(2-hydroxypropyl)-4,7,10-tris-((S
- Page 163 and 164: 7.11 Preparation of the silica mate
- Page 165 and 166: To a suspension of 33 (1.48 g, 1.1
- Page 167 and 168: gel-like suspension that was cooled
- Page 169 and 170: Si-GPS-Tri-Trac, 4545The title comp
- Page 171 and 172: APPENDIX ABINDING CONSTANT DETERMIN
- Page 173 and 174: 1.01000.880δ obs (ppm)0.60.4logK =
- Page 175 and 176: APPENDIX BDETERMINATION OF STOICHIO
- Page 177 and 178: 0.5χG∆δGFigure B.2χ GSimulated
- Page 179 and 180:
13 C CPMAS NMR SPECTRA OF SOME HIGH
- Page 181 and 182:
Si-GPS-[Cd(Trac)(2,5-diydroxybenzoa
- Page 183 and 184:
REFERENCES
- Page 185 and 186:
15. D. Tzalis and Y. Tor, Tetrahedr
- Page 187 and 188:
42. A. P. Davis and R. S.Wareham, A
- Page 189 and 190:
69. R. J. Bergeron, M. A. Channing
- Page 191 and 192:
93. C. B. Smith, A. K. W. Stephens,
- Page 193 and 194:
118. D. D. Dischino, E. J. Delaney,
- Page 195 and 196:
144. X. Huang, X. Chang, Q. He, Y.
- Page 197 and 198:
169. J. M. Klunder, T. Onami and K.
- Page 199 and 200:
197. K. A. Connors, Binding Constan
- Page 201 and 202:
221. J. Blumel, J. Am. Chem. Soc.,
- Page 203:
247. W. Likussar and D. F. Botz, An