Chemistry 155 Introduction to Instrumental Analytical Chemistry
Chemistry 155 Introduction to Instrumental Analytical Chemistry
Chemistry 155 Introduction to Instrumental Analytical Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
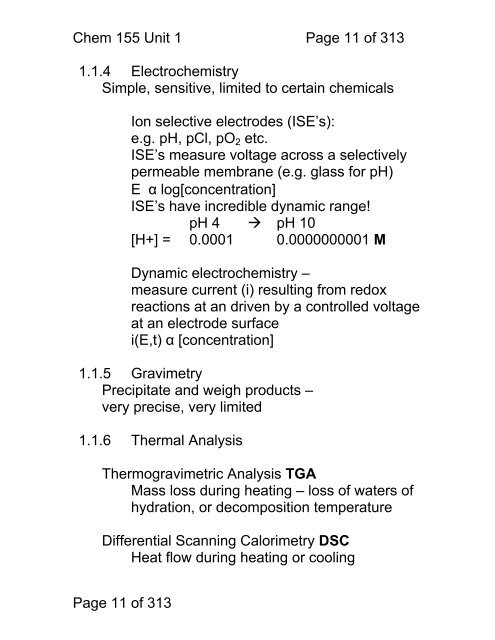
Chem <strong>155</strong> Unit 1 Page 11 of 3131.1.4 ElectrochemistrySimple, sensitive, limited <strong>to</strong> certain chemicalsIon selective electrodes (ISE’s):e.g. pH, pCl, pO 2 etc.ISE’s measure voltage across a selectivelypermeable membrane (e.g. glass for pH)E α log[concentration]ISE’s have incredible dynamic range!pH 4 pH 10[H+] = 0.0001 0.0000000001 MDynamic electrochemistry –measure current (i) resulting from redoxreactions at an driven by a controlled voltageat an electrode surfacei(E,t) α [concentration]1.1.5 GravimetryPrecipitate and weigh products –very precise, very limited1.1.6 Thermal AnalysisThermogravimetric Analysis TGAMass loss during heating – loss of waters ofhydration, or decomposition temperatureDifferential Scanning Calorimetry DSCHeat flow during heating or coolingPage 11 of 313