Chemistry 155 Introduction to Instrumental Analytical Chemistry
Chemistry 155 Introduction to Instrumental Analytical Chemistry
Chemistry 155 Introduction to Instrumental Analytical Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
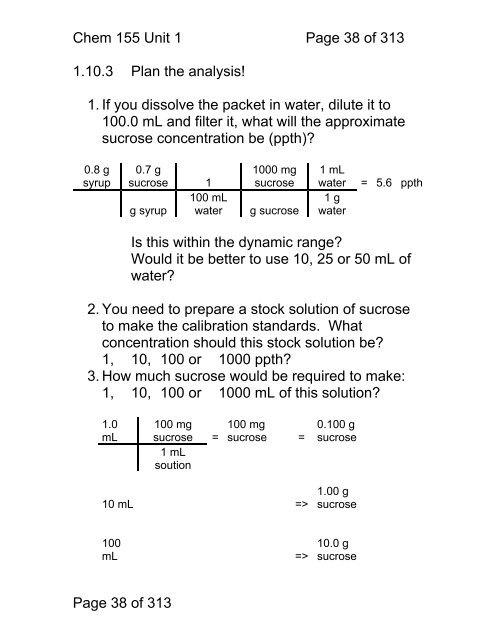
Chem <strong>155</strong> Unit 1 Page 38 of 3131.10.3 Plan the analysis!1. If you dissolve the packet in water, dilute it <strong>to</strong>100.0 mL and filter it, what will the approximatesucrose concentration be (ppth)?0.8 gsyrup0.7 gsucrose 1100 mLg syrup water1000 mgsucroseg sucrose1 mLwater1 gwater= 5.6 ppthIs this within the dynamic range?Would it be better <strong>to</strong> use 10, 25 or 50 mL ofwater?2. You need <strong>to</strong> prepare a s<strong>to</strong>ck solution of sucrose<strong>to</strong> make the calibration standards. Whatconcentration should this s<strong>to</strong>ck solution be?1, 10, 100 or 1000 ppth?3. How much sucrose would be required <strong>to</strong> make:1, 10, 100 or 1000 mL of this solution?1.0mL100 mgsucrose =1 mLsoution100 mgsucrose =0.100 gsucrose10 mL =>1.00 gsucrose100mL =>10.0 gsucrosePage 38 of 313