Chemistry 155 Introduction to Instrumental Analytical Chemistry
Chemistry 155 Introduction to Instrumental Analytical Chemistry
Chemistry 155 Introduction to Instrumental Analytical Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
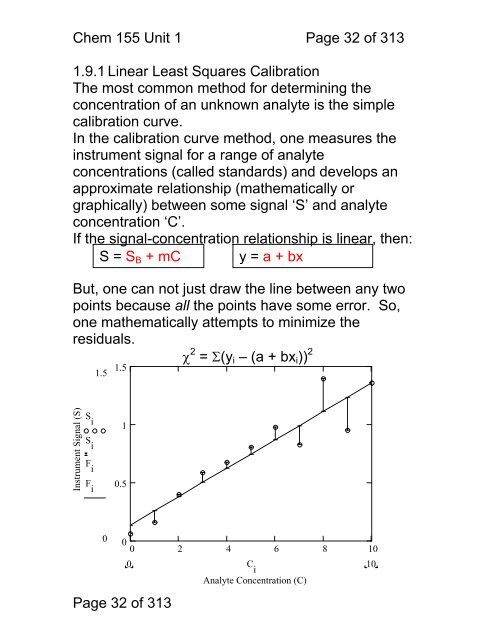
Chem <strong>155</strong> Unit 1 Page 32 of 3131.9.1 Linear Least Squares CalibrationThe most common method for determining theconcentration of an unknown analyte is the simplecalibration curve.In the calibration curve method, one measures theinstrument signal for a range of analyteconcentrations (called standards) and develops anapproximate relationship (mathematically orgraphically) between some signal ‘S’ and analyteconcentration ‘C’.If the signal-concentration relationship is linear, then:S = S B + mC y = a + bxBut, one can not just draw the line between any twopoints because all the points have some error. So,one mathematically attempts <strong>to</strong> minimize theresiduals.χ 2 = Σ(y i – (a + bx i )) 21.51.5Instrument Signal (S)S iS iF i10.5F i10000 2 4 6 8 10Page 32 of 3130 C iAnalyte Concentration (C)