INSTITUTUL NAÅ¢IONAL DE CERCETARE-DEZVOLTARE - ICECHIM
INSTITUTUL NAÅ¢IONAL DE CERCETARE-DEZVOLTARE - ICECHIM
INSTITUTUL NAÅ¢IONAL DE CERCETARE-DEZVOLTARE - ICECHIM
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
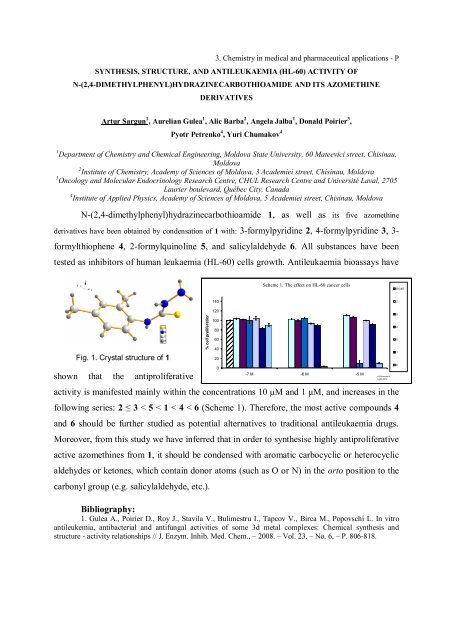
3. Chemistry in medical and pharmaceutical applications - PSYNTHESIS, STRUCTURE, AND ANTILEUKAEMIA (HL-60) ACTIVITY OFN-(2,4-DIMETHYLPHENYL)HYDRAZINECARBOTHIOAMI<strong>DE</strong> AND ITS AZOMETHINE<strong>DE</strong>RIVATIVESArtur Sargun 1 , Aurelian Gulea 1 , Alic Barba 2 , Angela Jalba 1 , Donald Poirier 3 ,Pyotr Petrenko 4 , Yuri Chumakov 41 Department of Chemistry and Chemical Engineering, Moldova State University, 60 Mateevici street, Chisinau,Moldova2 Institute of Chemistry, Academy of Sciences of Moldova, 3 Academiei street, Chisinau, Moldova3 Oncology and Molecular Endocrinology Research Centre, CHUL Research Centre and Université Laval, 2705Laurier boulevard, Québec City, Canada4 Institute of Applied Physics, Academy of Sciences of Moldova, 5 Academiei street, Chisinau, MoldovaN-(2,4-dimethylphenyl)hydrazinecarbothioamide 1, as well as its five azomethinederivatives have been obtained by condensation of 1 with: 3-formylpyridine 2, 4-formylpyridine 3, 3-formylthiophene 4, 2-formylquinoline 5, and salicylaldehyde 6. All substances have beentested as inhibitors of human leukaemia (HL-60) cells growth. Antileukaemia bioassays haveScheme 1. The effect on HL-60 cancer cellsDOXO1402Fig. 1. Crystal structure of 1% cell proliferation120100806040203451shown that the antiproliferative0-7 M -6 M -5 MAJ Protocole 504,05,20126activity is manifested mainly within the concentrations 10 μM and 1 μM, and increases in thefollowing series: 2 ≤ 3 < 5 < 1 < 4 < 6 (Scheme 1). Therefore, the most active compounds 4and 6 should be further studied as potential alternatives to traditional antileukaemia drugs.Moreover, from this study we have inferred that in order to synthesise highly antiproliferativeactive azomethines from 1, it should be condensed with aromatic carbocyclic or heterocyclicaldehydes or ketones, which contain donor atoms (such as O or N) in the orto position to thecarbonyl group (e.g. salicylaldehyde, etc.).Bibliography:1. Gulea A., Poirier D., Roy J., Stavila V., Bulimestru I., Tapcov V., Birca M., Popovschi L. In vitroantileukemia, antibacterial and antifungal activities of some 3d metal complexes: Chemical synthesis andstructure - activity relationships // J. Enzym. Inhib. Med. Chem., – 2008. – Vol. 23. – No. 6, – P. 806-818.