Druck-Materie 20b.qxd - JUWEL - Forschungszentrum Jülich
Druck-Materie 20b.qxd - JUWEL - Forschungszentrum Jülich
Druck-Materie 20b.qxd - JUWEL - Forschungszentrum Jülich
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
the formula also to the libration modes of around 170 (2) and 370 meV (3), and get the effective<br />
masses of 2.71 m and 3.13 m, respectively. They are also close to the free rotation effective<br />
mass. So, the recoil may be due to rotation.<br />
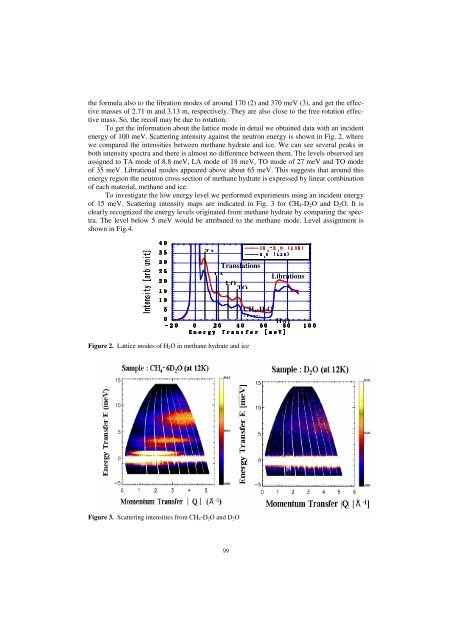
To get the information about the lattice mode in detail we obtained data with an incident<br />
energy of 100 meV. Scattering intensity against the neutron energy is shown in Fig. 2, where<br />
we compared the intensities between methane hydrate and ice. We can see several peaks in<br />
both intensity spectra and there is almost no difference between them. The levels observed are<br />
assigned to TA mode of 8.8 meV, LA mode of 18 meV, TO mode of 27 meV and TO mode<br />
of 35 meV. Librational modes appeared above about 65 meV. This suggests that around this<br />
energy region the neutron cross section of methane hydrate is expressed by linear combination<br />
of each material, methane and ice.<br />
To investigate the low energy level we performed experiments using an incident energy<br />
of 15 meV. Scattering intensity maps are indicated in Fig. 3 for CH4-D2O and D2O. It is<br />
clearly recognized the energy levels originated from methane hydrate by comparing the spectra.<br />
The level below 5 meV would be attributed to the methane mode. Level assignment is<br />
shown in Fig.4.<br />
¥ ¥ ¥ ¥<br />
¡ ¤ ¡ ¤ ¡ ¤ ¡ ¤<br />
¤ ¤ ¤ ¤<br />
¡ £ ¡ £ ¡ £ ¡ £<br />
£ £ £ £<br />
¡ ¢ ¡ ¢ ¡ ¢ ¡ ¢<br />
¢ ¢ ¢ ¢<br />
¡¡ ¡ ¡<br />
£ ¦ £ ¦ £ ¦ £ ¦<br />
TA<br />
Translations<br />
LA<br />
LO<br />
TO<br />
Figure 2. Lattice modes of H2O in methane hydrate and ice<br />
Figure 3. Scattering intensities from CH4-D2O and D2O<br />
£ £ £ ¥ ¥ ¥ ¥ § § § § ¨ ¨ ¨ ¨ ¢ ¢ ¢ ¢<br />
£<br />
� � � � � � � � � � � � � � � � � �<br />
� � � � � � � � � � � � � � � � � � �<br />
� � � � � � � � � � � � � � � � � � �<br />
� � � � � � � � � � � � � � � � � � �<br />
�<br />
99<br />
CH4-H2O<br />
(13K)<br />
� © � © � © � � �� � � � � � � � � � � �� � � � � � � � � � � � � � � � � � � � � � � � � �<br />
©<br />
�� � � �� � � � � � � � � � � � � � � � � � � � � � � � � � �<br />
Librations<br />
H2O