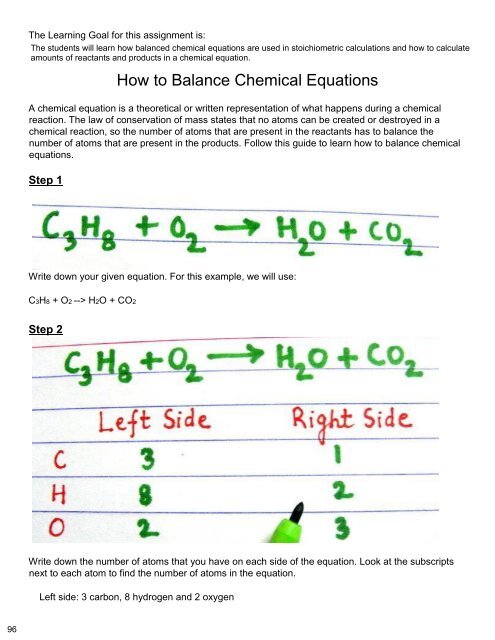
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Other atomic weights are listed on the periodic table (see our Periodic Table module). For each<br />
element listed, measuring out a quantity of the element equal to its atomic weight in grams will yield<br />
6.02 x 10 23 atoms of that element.<br />
The atomic weight of an element identifies both the mass of one mole of that element and the total<br />
number of protons and neutrons in an atom of that element. How can that be? Let's look at hydrogen.<br />
One mole of hydrogen atoms will weigh 1.01 grams.<br />
A hydrogen atom<br />
with its single electron<br />
Each hydrogen atom consists of one proton surrounded by one electron. But remember, the electron<br />
weighs so little that it does not contribute much to an atom's weight. Ignoring the weight of hydrogen's<br />
electrons, we can say that one mole of protons (H nuclei) weighs approximately one gram. Since<br />
protons and neutrons have about the same mass, a mole of either of these particles will weigh about<br />
one gram. For example, in one mole of helium, there are two moles of protons and two moles of<br />
neutrons - four grams of particles.<br />
Molecular weight<br />
If you stand on a scale with a friend, the scale will register the combined weight of both you and your<br />
friend. When atoms form molecules, the atoms bond together, and the molecule's weight is the<br />
combined weight of all of its parts.<br />
For example, every water molecule (H2O) has two atoms of hydrogen and one atom of oxygen. One<br />
mole of water molecules will contain two moles of hydrogen and one mole of oxygen.<br />
2 moles<br />
hydrogen<br />
Mole/weight relationships<br />
of water and its parts<br />
1 mole<br />
oxygen<br />
1 mole<br />
water<br />
+ =<br />
A bottle filled with exactly 18.02 g water will contain 6.02 x 10 23 water molecules. The concept of<br />
fractions and multiples described above also applies to molecules: 9.01 g of water would contain 1/2<br />
mole, or 3.01 x 10 23 molecules. You can calculate the molecular weight of any compound simply by<br />
summing the weights of atoms that make up that compound.<br />
93