You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
POLAR BONDING results when two different non-metals unequally share electrons between them.<br />
One well known exception to the identical atom rule is the combination of carbon and hydrogen in all<br />
organic compounds.<br />
The non-metal closer to fluorine in the Periodic Table has a greater tendency to keep its own electron<br />
and also draw away the other atom's electron. It is NOT completely successful. As a result, only<br />
partial charges are established. One atom becomes partially positive since it has lost control of its<br />
electron some of the time. The other atom becomes partially negative since it gains electron some of<br />
the time.<br />
Hydrogen Chloride<br />
Hydrogen Chloride forms a polar covalent molecule. The graphic<br />
on the left shows that chlorine has 7 electrons in the outer shell.<br />
Hydrogen has one electron in its outer energy shell. Since 8<br />
electrons are needed for an octet, they share the electrons.<br />
However, chlorine gets an unequal share of the two electrons,<br />
although the electrons are still shared (not transferred as in ionic<br />
bonding), the sharing is unequal. The electrons spends more of the<br />
time closer to chlorine. As a result, the chlorine acquires a "partial"<br />
negative charge. At the same time, since hydrogen loses the<br />
electron most - but not all of the time, it acquires a "partial" charge.<br />
The partial charge is denoted with a small Greek symbol for delta.<br />
Water<br />
Water, the most universal compound on all of the earth, has the property of<br />
being a polar molecule. As a result of this property, the physical and<br />
chemical properties of the compound are fairly unique.<br />
Dihydrogen Oxide or water forms a polar covalent molecule. The graphic on<br />
the left shows that oxygen has 6 electrons in the outer shell. Hydrogen has<br />
one electron in its outer energy shell. Since 8 electrons are needed for an<br />
octet, they share the electrons.<br />
Notes Section:<br />
There are two types of covalent bonding which are polar bonding and non polar. Non-polar bondingis when thiers is<br />
an equal sharing of electron. polarbonding and unequal sharing electrons and number of shared electrons matter<br />
on theoctect rule to become stable. non polar is when two same not metals share elctrons. Their only one thing from this<br />
which is hydrogen.<br />
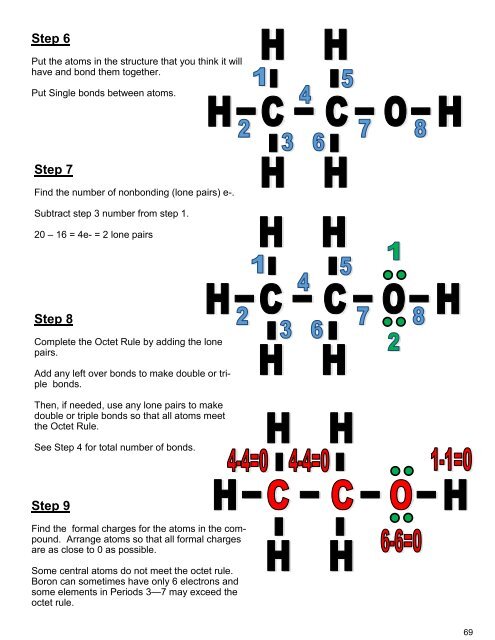
In order to make the covalent bond is go through the 8 step procces which is First of is you need to find the valence electron<br />
for all atoms. Add them together. Next we need to find octet for each atom and add them together. Furthermore (3) we<br />
need to Subtract Step 1 from Step 2. Find number of bonds by diving the number by 2 which gives us the bond.<br />
Additionaly we find out which is least electronegative is. Then we put the atoms in the structure that bond them together.<br />
Use the periodic table and find the one farthest away from Fluorine .Then we subtratct step 1from 3 and divide by 2 to get<br />
number of Lone pair. <strong>Final</strong>ly we find formal charges for the atoms. Arrange atoms so that all formal charges are 0 ot<br />
Ex: H20t<br />
Metal: non metal 1 1 to 1 ratio. When two metals come togethere they disacioate . Spread all over the place,<br />
1. H2*1=2 In this step we look at the group . For exampl hydrogen is 1 and oxegen is in group 6.<br />
O1*6 =6<br />
8e-<br />
2. H2*2=4 In the second step we Multiply all things by eight to satisfy the octet rule of eight. The inly execption<br />
O1*8=8 Hydrogen which you multiply by .<br />
12e-<br />
3 12-6= 6e All you do in this tep is subtract step 2 by step 1.<br />
4 6/2 -= 3bonds All we do in this step as you can clearly can see is divide eby 2 to get the number of bond we need.<br />
5And 6<br />
We ajusted this to a structure in which makes sense<br />
H O H<br />
7&8 8-6=2 lp We plug 2 lone pair on top<br />
Prefix number<br />
Mono 1<br />
Di 2<br />
tri 3<br />
tetra 4<br />
pentra 5<br />
hexa 6<br />
hepta 7<br />
octa 8<br />
nona 9<br />
deca 10 67