Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
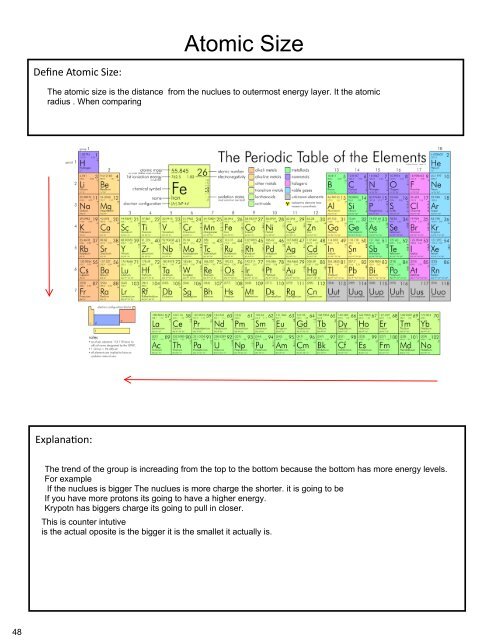
The Learning Goal for this Assignment is<br />
Restate properties of atoms and their postioms in the periodic table to the arragment of thier electrons.<br />
Alkali Metals<br />
They are a group in the periodic table consisting of the chemical elements lithium, sodium, pottasium, rubidium, caesium<br />
and francium. This group lies in th s-block of the periodoc table of elements as all alakli elements as they all have thier<br />
outermost electron in the s-orbital<br />
Alkali Earth Metals<br />
They are six chemical elements in group 2 of the periodic table. They all have similar properties such as<br />
bieng shiny, silvery white, somewhat reactive metals at standard temperature and presure.<br />
Beryllium, magnesium, calcium, stronium, barium, radium<br />
Transitional Metals<br />
Transitional metal elements are elements whose atoms has a partially filled d sub-shell or which can give rise to cations<br />
with an incomplete d sub-shell. They are from partial of group 3 through group 12.<br />
Inter Transitional Metals<br />
They are one of a group of chemical elements on the periodic table. They are normally shown in two rows below<br />
all the other elements on the periodic tablel They iclude elements 57 through 71 and 89 through 103.<br />
They have 3 complete outermost electron shells and are all metals. Sometimes are malleable and ductile.<br />
Metals<br />
I<br />
It is an element that is typically hard,shiny, and is a good at conducting electricity and thermal cundictivity. They are<br />
malleable they can be hammered without breaking and are as well fussible. 91 of the 118 elements are metals.<br />
Metalloids<br />
metalloid is any chemical element which has properties in between of metals and nonmetals or that has a<br />
mixture of them. Typical metalloids have a metallic appearance, but they are brittle and are okay conductors of<br />
electricity. They behave mostly as nonmetals<br />
Non Metals<br />
Thwy mostly lacks metallic. Nonmetals tend to be easily vaporized have low elasticity and are good insulators of heat<br />
and electricity. They have high ionization energy and electronegativity . Seventeen elements are non metals which are<br />
mostly gases.<br />
Noble Gases<br />
They make up a group of chemical elements with similar properties which are that they are all odorless, colorless,<br />
monatomic gases with very low chemical reactivity. The seven noble gases are helium, neon, argon,<br />
krypton , xenon, tradon. and Oganesson.<br />
46