Etude par Sonde Atomique Tomographique de la formation de nano ...
Etude par Sonde Atomique Tomographique de la formation de nano ...
Etude par Sonde Atomique Tomographique de la formation de nano ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
tel-00751814, version 1 - 14 Nov 2012<br />
Chapter 3. Oxi<strong>de</strong> Dispersion Strengthened Steels<br />
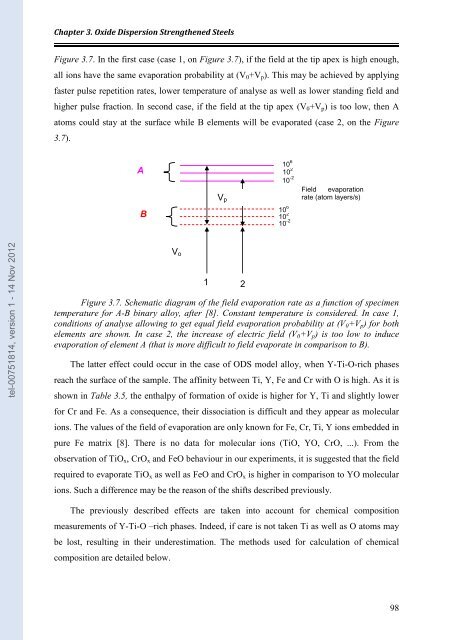
Figure 3.7. In the first case (case 1, on Figure 3.7), if the field at the tip apex is high enough,<br />
all ions have the same evaporation probability at (V0+Vp). This may be achieved by applying<br />
faster pulse repetition rates, lower temperature of analyse as well as lower standing field and<br />
higher pulse fraction. In second case, if the field at the tip apex (V0+Vp) is too low, then A<br />
atoms could stay at the surface while B elements will be evaporated (case 2, on the Figure<br />
3.7).<br />
A<br />
B<br />
Figure 3.7. Schematic diagram of the field evaporation rate as a function of specimen<br />
temperature for A-B binary alloy, after [8]. Constant temperature is consi<strong>de</strong>red. In case 1,<br />
conditions of analyse allowing to get equal field evaporation probability at (V0+Vp) for both<br />
elements are shown. In case 2, the increase of electric field (V0+Vp) is too low to induce<br />
evaporation of element A (that is more difficult to field evaporate in com<strong>par</strong>ison to B).<br />
The <strong>la</strong>tter effect could occur in the case of ODS mo<strong>de</strong>l alloy, when Y-Ti-O-rich phases<br />
reach the surface of the sample. The affinity between Ti, Y, Fe and Cr with O is high. As it is<br />
shown in Table 3.5, the enthalpy of <strong>formation</strong> of oxi<strong>de</strong> is higher for Y, Ti and slightly lower<br />
for Cr and Fe. As a consequence, their dissociation is difficult and they appear as molecu<strong>la</strong>r<br />
ions. The values of the field of evaporation are only known for Fe, Cr, Ti, Y ions embed<strong>de</strong>d in<br />
pure Fe matrix [8]. There is no data for molecu<strong>la</strong>r ions (TiO, YO, CrO, ...). From the<br />
observation of TiOx, CrOx and FeO behaviour in our experiments, it is suggested that the field<br />
required to evaporate TiOx as well as FeO and CrOx is higher in com<strong>par</strong>ison to YO molecu<strong>la</strong>r<br />
ions. Such a difference may be the reason of the shifts <strong>de</strong>scribed previously.<br />
The previously <strong>de</strong>scribed effects are taken into account for chemical composition<br />
measurements of Y-Ti-O –rich phases. In<strong>de</strong>ed, if care is not taken Ti as well as O atoms may<br />
be lost, resulting in their un<strong>de</strong>restimation. The methods used for calcu<strong>la</strong>tion of chemical<br />
composition are <strong>de</strong>tailed below.<br />
Vo<br />
Vp<br />
1 2<br />
10 6<br />
10 2<br />
10 -2<br />
10 -2<br />
10 2<br />
10 6<br />
Field evaporation<br />
rate (atom <strong>la</strong>yers/s)<br />
98