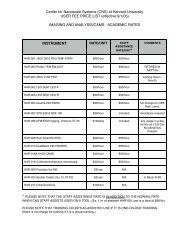
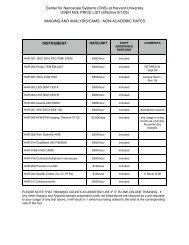
Atomic Layer Deposition (ALD): An Enabler for Nanoscience and ...
Atomic Layer Deposition (ALD): An Enabler for Nanoscience and ...
Atomic Layer Deposition (ALD): An Enabler for Nanoscience and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>): <strong>An</strong> <strong>Enabler</strong><br />
<strong>for</strong> <strong>Nanoscience</strong> <strong>and</strong> Nanotechnology<br />
Harvard University<br />
Roy G. Gordon<br />
Harvard University<br />
Cambridge, MA
Definitions of Chemical Vapor <strong>Deposition</strong> (CVD)<br />
<strong>and</strong> <strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>)<br />
Structures <strong>and</strong> materials made by <strong>ALD</strong><br />
Properties needed <strong>for</strong> CVD <strong>and</strong> <strong>ALD</strong> precursors:<br />
Volatility, Stability, Reactivity<br />
How to design those properties into precursors:<br />
metal amidinates<br />
High-k insulators: La 2 O 3 , LaAlO 3<br />
Harvard University<br />
Outline
Chemical Vapor <strong>Deposition</strong> (CVD)<br />
One or more gases or vapors react to <strong>for</strong>m a solid product<br />
precursor<br />
vapors<br />
substrate<br />
Solid product can be a<br />
film<br />
particle<br />
nanowire<br />
nanotube<br />
Harvard University<br />
Heater<br />
film<br />
byproduct<br />
vapors<br />
Reaction started by<br />
heat<br />
mixing 2 vapors<br />
plasma
<strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>)<br />
Sequential, self-limiting surface reactions make alternating layers:<br />
Precursor 1<br />
Precursor 2<br />
Benefits of <strong>ALD</strong>:<br />
• <strong>Atomic</strong> level of control over film composition<br />
⇒nanolaminates <strong>and</strong> multi-component materials<br />
• Uni<strong>for</strong>m thickness over large areas <strong>and</strong> inside narrow holes<br />
• Very smooth surfaces (<strong>for</strong> amorphous films)<br />
• High density <strong>and</strong> few defects or pinholes<br />
• Low deposition temperatures (<strong>for</strong> very reactive precursors)<br />
• Pure films (<strong>for</strong> suitably reactive precursors)<br />
Harvard University<br />
Heated area =<br />
deposition zone
ML 2<br />
H 2O<br />
Typical <strong>ALD</strong> Reaction <strong>for</strong> Oxides<br />
ML 2<br />
ML 2<br />
OH OH OH<br />
ML<br />
O<br />
H 2O<br />
ML<br />
O<br />
Harvard University<br />
H 2O<br />
ML<br />
O<br />
ML<br />
O<br />
MOH<br />
O<br />
HL<br />
ML<br />
O<br />
HL<br />
MOH<br />
O<br />
HL<br />
ML<br />
O<br />
HL<br />
MOH<br />
O<br />
HL<br />
HL
Definitions of Chemical Vapor <strong>Deposition</strong> (CVD)<br />
<strong>and</strong> <strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>)<br />
Structures <strong>and</strong> materials made by <strong>ALD</strong><br />
Properties needed <strong>for</strong> CVD <strong>and</strong> <strong>ALD</strong> precursors:<br />
Volatility, Stability, Reactivity<br />
How to design those properties into precursors:<br />
metal amidinates<br />
High-k insulators: La 2 O 3 , LaAlO 3<br />
Harvard University<br />
Outline
Lining <strong>and</strong> Filling Holes by <strong>ALD</strong><br />
Harvard University<br />
4 cycles 12 cycles
Ion Milling<br />
Ion Milling + <strong>ALD</strong><br />
Harvard University<br />
Nanopores by <strong>ALD</strong><br />
May be used <strong>for</strong> rapid sequencing of DNA
Coatings on the Outside of Particles<br />
<strong>ALD</strong> AlN coating<br />
ZnS particles<br />
Used in electroluminescent back-lights <strong>for</strong> displays<br />
in cell-phones <strong>and</strong> many other devices.<br />
Harvard University
Harvard University<br />
Photonic Crystals by <strong>ALD</strong><br />
1) Form crystals of silica spheres.<br />
2) <strong>ALD</strong> of Ta 2 O 5 between the spheres<br />
3) Convert to Ta 3 N 5 in NH 3<br />
4) Dissolve the silica spheres in HF<br />
f<br />
500nm<br />
The resulting photonic crystals may be able to control light,<br />
the way semiconductors control electron transport.
<strong>ALD</strong> ruthenium<br />
on aluminum oxide<br />
diameters ~1 to 2 nm,<br />
~ 5 to 10 atoms across<br />
nucleation density is<br />
~ 20 x higher than previous<br />
metal nanocrystals<br />
may be applied to<br />
flash memories<br />
Harvard University<br />
Nano-Dots by <strong>ALD</strong><br />
40 nm scale bar; 10 nm in insert
after 500 cycles of Al 2 O 3<br />
Harvard University<br />
Nanobeads by <strong>ALD</strong><br />
Growth on Single-walled Carbon Nanotubes<br />
after 500 cycles of iron
Alumina Nanotubes on Carbon Nanotubes<br />
7 nm diameter<br />
Harvard University<br />
21 nm diameter<br />
100 nm diameter
Nano-Coaxial Cable or Transistor<br />
Conducting tungsten nitride (WN) concentrically around<br />
insulating aluminum oxide (Al 2 O 3 ) concentrically around<br />
a conducting carbon nanotube.<br />
WN<br />
Harvard University<br />
Al 2 O 3<br />
Carbon Al 2 O 3 WN
Elements included in <strong>ALD</strong> Materials<br />
Green = Element included in at least 1 <strong>ALD</strong> Material<br />
Red = Element not included in any <strong>ALD</strong> Material<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr<br />
Harvard University
Harvard University<br />
Oxides Made by <strong>ALD</strong><br />
Green = <strong>ALD</strong> process known <strong>for</strong> an Oxide of the Element<br />
Red = no process known <strong>for</strong> <strong>ALD</strong> of any Oxide of the Element<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Pure Elements Made by <strong>ALD</strong><br />
Green = <strong>ALD</strong> processes known <strong>for</strong> 16 Pure Elements<br />
Red = no process known <strong>for</strong> <strong>ALD</strong> of the Element<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr<br />
Harvard University
Harvard University<br />
Nitrides Made by <strong>ALD</strong><br />
Green = <strong>ALD</strong> processes known <strong>for</strong> a Nitride of the Element<br />
Red = no process known <strong>for</strong> <strong>ALD</strong> of a Nitride of the Element<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Harvard University<br />
Sulfides Made by <strong>ALD</strong><br />
Green = <strong>ALD</strong> processes known <strong>for</strong> a Sulfide of the Element<br />
Red = no process known <strong>for</strong> <strong>ALD</strong> of a Sulfide of the Element<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Harvard University<br />
Carbides Made by <strong>ALD</strong><br />
Green = <strong>ALD</strong> processes known <strong>for</strong> a Carbide of the Element<br />
Red = no process known <strong>for</strong> <strong>ALD</strong> of a Carbide of the Element<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Harvard University<br />
Fluorides Made by <strong>ALD</strong><br />
Green = <strong>ALD</strong> processes known <strong>for</strong> a Fluoride of the Element<br />
Red = no process known <strong>for</strong> <strong>ALD</strong> of a Fluoride of the Element<br />
H He<br />
Li Be B C N O F Ne<br />
Na Mg Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn<br />
Fr Ra Ac<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Current Applications of <strong>ALD</strong><br />
Electroluminescent displays (Al 2 O 3 , AlN, ZnS)<br />
Read/Write heads in magnetic disk storage (Al 2 O 3 )<br />
Insulators in capacitors in DRAMs (Al 2 O 3 , HfO 2 )<br />
Insulation <strong>and</strong> spacer layers in microelectronics (SiO 2 , Si 3 N 4 )<br />
Metal/insulator in transistor gates (TaN/HfO 2)<br />
Planar waveguides <strong>and</strong> optical filters (SiO 2 , TiO 2 )<br />
Likely Future Applications of <strong>ALD</strong><br />
Insulators in microelectronic capacitors (Ta 2 O 5 , SrTiO 3 , LaLuO 3 )<br />
Diffusion barriers <strong>for</strong> copper in interconnects (WN, TaN, Mn)<br />
Adhesion <strong>and</strong> seed layers <strong>for</strong> interconnects (Co 4 N, Ru, Cu)<br />
Sealing pores in low-k dielectrics (SiO 2 )<br />
Magnetic disk storage (Al 2 O 3 , Fe, Co, Ni, Cu, Ru, Mn, Pt)<br />
Nano-Electronics<br />
Catalysts . . .<br />
Harvard University<br />
Applications of <strong>ALD</strong>
Definitions of Chemical Vapor <strong>Deposition</strong> (CVD)<br />
<strong>and</strong> <strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>)<br />
Structures <strong>and</strong> materials made by <strong>ALD</strong><br />
Properties needed <strong>for</strong> CVD <strong>and</strong> <strong>ALD</strong> precursors:<br />
Volatility, Stability, Reactivity<br />
How to design those properties into precursors:<br />
metal amidinates<br />
High-k insulators: La 2 O 3 , LaAlO 3<br />
Harvard University<br />
Outline
Criteria <strong>for</strong> Both CVD & <strong>ALD</strong> Precursors<br />
•Sufficient volatility (> 0.1 Torr at T < 200 o C)<br />
•No thermal decomposition during vaporization<br />
•Liquid at vaporization temperature<br />
•Preferably liquid at room temperature<br />
or soluble in an inert solvent<br />
•Precursors <strong>and</strong> byproducts don’t etch films<br />
Harvard University
Criteria <strong>for</strong> CVD Precursors<br />
•Reactivity with substrate<br />
•Reactivity with surface of growing film<br />
•Thermal decomposition allowed or even needed<br />
Harvard University<br />
Criteria <strong>for</strong> <strong>ALD</strong> Precursors<br />
•Self-limited reactivity with substrate<br />
•Self-limited reactivity with the surface made by<br />
reaction of the film with the other precursor<br />
•Thermal decomposition not allowed
Usefulness of Precursors <strong>for</strong> CVD & <strong>ALD</strong><br />
Some precursors work only in CVD, but not <strong>ALD</strong>:<br />
Ni(CO) 4 , W(CO) 6 , many alkoxides<br />
Some precursors work in both CVD <strong>and</strong> <strong>ALD</strong>:<br />
many beta-diketonates <strong>and</strong> amidinates<br />
Most <strong>ALD</strong> precursors<br />
also work in CVD<br />
Some CVD precursors<br />
also work in <strong>ALD</strong><br />
Harvard University<br />
CVD<br />
<strong>ALD</strong>
Definitions of Chemical Vapor <strong>Deposition</strong> (CVD)<br />
<strong>and</strong> <strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>)<br />
Structures <strong>and</strong> materials made by <strong>ALD</strong><br />
Properties needed <strong>for</strong> CVD <strong>and</strong> <strong>ALD</strong> precursors:<br />
Volatility, Stability, Reactivity<br />
How to design those properties into precursors:<br />
metal amidinates<br />
High-k insulators: La 2 O 3 , LaAlO 3<br />
Harvard University<br />
Outline
Acetamidinates: R2 Formamidinates: R<br />
= CH3 2 = H<br />
R 2<br />
R 1<br />
N<br />
N<br />
R 3<br />
M<br />
Harvard University<br />
Metal(II) Amidinates<br />
Propionamidinates: R 2 = CH 2 CH 3<br />
R 1<br />
N<br />
N<br />
R 3<br />
R 2<br />
R 1 <strong>and</strong> R 3 = alkyl groups<br />
monomer dimer<br />
R 2<br />
R 3 R1 R 3<br />
R 1<br />
N<br />
N<br />
R 3<br />
N N<br />
M<br />
M<br />
N N<br />
M-N bonds are generally reactive to H 2 O, NH 3 , H 2 , etc.<br />
The chelate structure adds to the thermal stability.<br />
The choices of R n affect the volatility, reactivity <strong>and</strong> stability.<br />
R 2<br />
R 2<br />
N<br />
N<br />
R 1<br />
R 1<br />
R 3<br />
R 2
Increasing lig<strong>and</strong> bulk<br />
Structures of Metal Bis-Acetamidinates<br />
tert-pentyl 2<br />
tert-butyl 2<br />
isopropyl 2<br />
Et-tert-Bu<br />
n-propyl 2<br />
R 1 , R 3<br />
ionic radius<br />
Harvard University<br />
c<br />
m<br />
m<br />
Ni<br />
69<br />
c<br />
m<br />
d<br />
d<br />
Co<br />
65<br />
c<br />
m<br />
d<br />
Cr<br />
73<br />
m<br />
Ge<br />
73<br />
m<br />
Zn<br />
74<br />
m<br />
d<br />
Mg<br />
72<br />
m<br />
d<br />
Fe<br />
78<br />
Increasing “size” of metal atom<br />
Mn<br />
c = crowded, less reactive, more stable, volatile monomer<br />
d<br />
d<br />
83<br />
m<br />
Bi<br />
103<br />
m<br />
d<br />
Ca<br />
100<br />
m = more reactive, less stable, volatile monomer<br />
m<br />
d = reactive, volatile dimer<br />
d<br />
d<br />
d<br />
p<br />
p<br />
Sr<br />
118<br />
d<br />
d<br />
p<br />
p<br />
Ba<br />
135<br />
p = non-volatile polymer
R 2<br />
R 1<br />
N N<br />
R3 R1 N<br />
R 2<br />
C<br />
M<br />
C N N C<br />
R 1 R 3<br />
Harvard University<br />
Metal(III) Amidinates<br />
N<br />
monomer<br />
R 3<br />
R 2<br />
Formamidinates: R 2 = H<br />
Acetamidinates: R 2 = CH 3<br />
Propionamidinates: R 2 = CH 2 CH 3<br />
R 1 <strong>and</strong> R 3 = alkyl groups<br />
Structures of dimers are unknown, probably bridged
Increasing lig<strong>and</strong> bulk<br />
Structures of Metal(III) Tris-Amidinates<br />
tert-<br />
Bu 2<br />
iso-<br />
Pr 2<br />
Et-<br />
t Bu<br />
n-Pr 2<br />
Et 2<br />
Me 2<br />
R 1 ,R 3<br />
c<br />
Al<br />
c<br />
Ga<br />
n<br />
c<br />
c<br />
m<br />
m<br />
Cr<br />
Harvard University<br />
c<br />
Co<br />
c<br />
V<br />
c<br />
Fe<br />
n<br />
c<br />
m<br />
m<br />
d<br />
Ti<br />
m<br />
Ru<br />
m<br />
m<br />
d<br />
Sc<br />
m<br />
Sb<br />
Increasing size of metal atom<br />
n = non-existent<br />
c = crowded, less reactive monomer<br />
c<br />
c<br />
c<br />
c<br />
m<br />
m<br />
m<br />
Lu<br />
c<br />
m<br />
d<br />
Y<br />
m<br />
Gd<br />
c<br />
Eu<br />
c<br />
Nd<br />
m = more reactive monomer<br />
d = low-volatility dimer<br />
m<br />
Pr<br />
c<br />
Ce<br />
c<br />
m<br />
d<br />
p<br />
p<br />
La<br />
p = non-volatile polymer
Models <strong>for</strong> Lanthanum <strong>and</strong> Sc<strong>and</strong>ium Amidinates<br />
La ion is large, so 3 amidinate<br />
lig<strong>and</strong>s are not crowded<br />
La<br />
La precursor reacts<br />
quickly with surface OH<br />
Harvard University<br />
Sc ion is small, so 3 amidinate<br />
lig<strong>and</strong>s are crowded<br />
Sc<br />
Sc precursor reacts<br />
slowly with surface OH
Zr(amd) 4 <strong>and</strong> Hf(amd) 4<br />
Harvard University<br />
Metal(IV) Tetra-Amidinates<br />
Require small R n groups<br />
such as H <strong>and</strong> CH 3<br />
H 3C<br />
H<br />
H 3C N<br />
H<br />
N<br />
CH 3<br />
N<br />
N<br />
Hf<br />
CH 3<br />
CH 3<br />
N<br />
N<br />
CH 3<br />
H<br />
N CH 3<br />
N<br />
H<br />
CH 3<br />
Thermal decomposition at 200 o C<br />
Zr amidinate is much more stable<br />
than Zr amide, Zr(NEtMe) 4<br />
Greater stability of amidinates is<br />
due to chelate structure (2 metalnitrogen<br />
bonds instead of one)
Definitions of Chemical Vapor <strong>Deposition</strong> (CVD)<br />
<strong>and</strong> <strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> (<strong>ALD</strong>)<br />
Structures <strong>and</strong> materials made by <strong>ALD</strong><br />
Properties needed <strong>for</strong> CVD <strong>and</strong> <strong>ALD</strong> precursors:<br />
Volatility, Stability, Reactivity<br />
How to design those properties into precursors:<br />
metal amidinates<br />
High-k insulators: La 2 O 3 , LaAlO 3<br />
Harvard University<br />
Outline
Harvard University<br />
Thermogravimetric <strong>An</strong>alysis of<br />
Lanthanum Amidinates<br />
N<br />
N<br />
H<br />
C N<br />
N<br />
HC<br />
La<br />
H 3C<br />
N<br />
CH<br />
N<br />
N C N<br />
N<br />
C<br />
La<br />
N<br />
CH 3<br />
N<br />
C<br />
N<br />
CH 3<br />
CH 3<br />
N C N<br />
N N<br />
C<br />
N<br />
C<br />
N<br />
La<br />
H3C CH3 H3C H3C => Vaporization temperature increases with molecular mass<br />
CH 3<br />
CH 3<br />
CH 3<br />
CH 3
Vapor Pressures of Lanthanum Precursors<br />
=> La(iPr 2 -fmd) 3 is most<br />
volatile La compound known,<br />
60 mTorr at 100 o C<br />
Harvard University<br />
N<br />
H<br />
C N<br />
N<br />
HC<br />
N<br />
La<br />
N<br />
N<br />
CH<br />
La(iPrCp) 3<br />
0.1<br />
Torr
Precursors:<br />
H 2 O <strong>and</strong><br />
Harvard University<br />
<strong>ALD</strong> of La 2 O 3<br />
tris(N,N’-diisopropyl<strong>for</strong>mamidinato)lanthanum<br />
( i Pr 2 -fmd) 3 La<br />
N<br />
H<br />
C N<br />
N<br />
HC<br />
N<br />
La<br />
N<br />
N<br />
CH<br />
=> 0.16 nm per cycle<br />
=> negligible delay<br />
in nucleation on SiH
Growth per La Cycle <strong>for</strong> <strong>ALD</strong> LaAlO 3<br />
Precursors:<br />
Me 3 Al, H 2 O <strong>and</strong><br />
90 o C<br />
Harvard University<br />
tris(N,N’-diisopropyl<strong>for</strong>mamidinato)lanthanum<br />
( i Pr 2 -fmd) 3 La<br />
110 o C<br />
100 o C<br />
120 o C<br />
Bubbler temperature 90 to 120 o C<br />
Substrate temperature 300 o C<br />
=> <strong>ALD</strong> saturation at<br />
0.08 nm per La cycle<br />
Growth even at bubbler<br />
temperature
Precursors:<br />
Me 3 Al, H 2 O <strong>and</strong><br />
Harvard University<br />
Composition of <strong>ALD</strong> La xAl 1-xO 3/2<br />
tris(N,N’-diisopropyl<strong>for</strong>mamidinato)lanthanum<br />
( i Pr 2 -fmd) 3 La<br />
Growth conditions:<br />
Bubbler temperature 120 o C<br />
Substrate temperature 300 o C<br />
=> Composition control<br />
by changing ratio of<br />
precursor doses<br />
=> 2 x as many Al atoms<br />
as La atoms per dose<br />
N<br />
H<br />
C N<br />
N<br />
HC<br />
N<br />
La<br />
N<br />
N<br />
CH
Precursor Reactivity with SiH Surface by IR<br />
La amidinate reacts<br />
with nearly all the<br />
Si-H bonds in only<br />
3 cycles<br />
Hf alkylamide only<br />
reacts with half of<br />
the Si-H bonds on<br />
the surface even<br />
after many cycles<br />
=> Completely uni<strong>for</strong>m surface coverage by La amidinate<br />
Details of the infrared data were given at AVS Conference <strong>ALD</strong> 2007 by<br />
J. Kwon, M. Dai, E. Langereis, Y. Chabal, K.-H. Kim <strong>and</strong> R. G. Gordon.<br />
Harvard University
2 nm<br />
TEMs of <strong>ALD</strong> LaAlO 3 <strong>and</strong> GdScO 3<br />
=> Sharp interfaces with silicon without interlayers<br />
=> Uni<strong>for</strong>m nucleation <strong>and</strong> thickness<br />
Harvard University<br />
LaAlO 3<br />
Si
Leakage Current through <strong>ALD</strong> La 2 O 3<br />
Vapor source: a solution of the La precursor (mp 194 o C)<br />
vaporized with an MKS MDD liquid delivery system<br />
Low leakage current similar to films made from a bubbler.<br />
=> negligible carbon contamination from solvent<br />
Harvard University
Leakage (A/cm 2 )<br />
10 2<br />
10 0<br />
10 -2<br />
10 -4<br />
10 -6<br />
10 -8<br />
Harvard University<br />
Comparison of leakage<br />
SiO 2<br />
<strong>ALD</strong> LaAlO 3<br />
<strong>ALD</strong> GdScO 3<br />
0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0<br />
EOT (nm, |V g -V fb |=1V)<br />
<strong>ALD</strong> LaScO 3<br />
<strong>ALD</strong> HfO 2 from IMEC (Ref.1)<br />
MOCVD HfO 2 from IMEC (Ref.2)<br />
<strong>ALD</strong> HfO 2 from IBM (Ref.3)<br />
Sputter HfO 2 (Ref.4)
Harvard University<br />
Summary<br />
<strong>ALD</strong> requires volatile precursors with self-limited reactivity<br />
<strong>and</strong> high thermal stability<br />
Precursors with these properties are known <strong>for</strong> most<br />
elements<br />
<strong>ALD</strong> is a proven process with many current applications<br />
Many more uses <strong>for</strong> <strong>ALD</strong> are expected in the future
Harvard University<br />
Acknowledgements<br />
Metals: Booyong Lim, <strong>An</strong>tti Rahtu, Jin-Seong Park, Venkateswara Pallem<br />
Cu, Co: Zhengwen Li, Séan Barry, Don Keun Lee, Harish Bh<strong>and</strong>ari, Hoon Kim<br />
Ruthenium: Huazhi Li, Titta Aaltonen, Jun Ni<br />
Metal Nitrides: Jill Becker, Seigi Suh, Esther Kim, Kyoung-ha Kyoung ha Kim<br />
Metal oxides: Dennis Hausmann, Philippe de Rouffignac, Jin-Seong Park,<br />
Kyoung-ha Kyoung ha Kim, Leo Rodriguez, Mike Coulter, Jean Sébastien Lehn, Sheng<br />
Xu, Hongtao Wang, Yiqun Liu<br />
TEM: Damon Farmer; Hongtao Wang, SEMATECH, Applied Materials<br />
DRAM trenches supplied by Infineon (Qimonda)<br />
La, Co, Cu <strong>and</strong> Ru precursors supplied by Rohm <strong>and</strong> Haas Electronic Materials<br />
SiO 2 <strong>and</strong> W precursors supplied by Sigma-Aldrich Company<br />
SEMs <strong>and</strong> Electrical <strong>An</strong>alysis by Daniel Josell, NIST<br />
Supported by the US National Science Foundation <strong>and</strong> Intel Corporation