Hyperbaric Oxygen Therapy - Hyperbaric Chamber Information ...
Hyperbaric Oxygen Therapy - Hyperbaric Chamber Information ...
Hyperbaric Oxygen Therapy - Hyperbaric Chamber Information ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
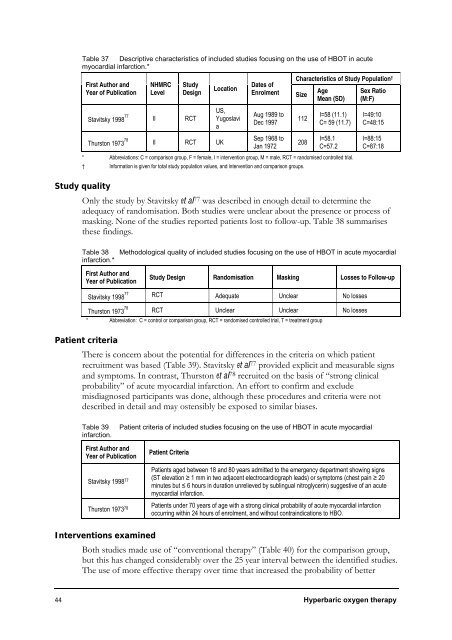
Table 37 Descriptive characteristics of included studies focusing on the use of HBOT in acute<br />
myocardial infarction.*<br />
First Author and<br />
Year of Publication<br />
Stavitsky 1998 77<br />
Thurston 1973 78<br />
NHMRC<br />
Level<br />
Study<br />
Design<br />
II RCT<br />
II RCT UK<br />
Location<br />
US,<br />
Yugoslavi<br />
a<br />
Dates of<br />
Enrolment Size<br />
Aug 1989 to<br />
Dec 1997<br />
Sep 1968 to<br />
Jan 1972<br />
Characteristics of Study Population †<br />
Age<br />
Mean (SD)<br />
Sex Ratio<br />
(M:F)<br />
44 <strong>Hyperbaric</strong> oxygen therapy<br />
112<br />
208<br />
I=58 (11.1)<br />
C= 59 (11.7)<br />
I=58.1<br />
C=57.2<br />
* Abbreviations: C = comparison group, F = female, I = intervention group, M = male, RCT = randomised controlled trial.<br />
† <strong>Information</strong> is given for total study population values, and intervention and comparison groups.<br />
I=49:10<br />
C=48:15<br />
I=88:15<br />
C=87:18<br />
Study quality<br />
Only the study by Stavitsky et al77 was described in enough detail to determine the<br />
adequacy of randomisation. Both studies were unclear about the presence or process of<br />
masking. None of the studies reported patients lost to follow-up. Table 38 summarises<br />
these findings.<br />
Table 38 Methodological quality of included studies focusing on the use of HBOT in acute myocardial<br />
infarction.*<br />
First Author and<br />
Year of Publication<br />
Study Design Randomisation Masking Losses to Follow-up<br />
Stavitsky 1998 77 RCT Adequate Unclear No losses<br />
Thurston 1973 78<br />
RCT Unclear Unclear No losses<br />
* Abbreviation: C = control or comparison group, RCT = randomised controlled trial, T = treatment group<br />
Patient criteria<br />
There is concern about the potential for differences in the criteria on which patient<br />
recruitment was based (Table 39). Stavitsky et al 77 provided explicit and measurable signs<br />
and symptoms. In contrast, Thurston et al 78 recruited on the basis of “strong clinical<br />
probability” of acute myocardial infarction. An effort to confirm and exclude<br />
misdiagnosed participants was done, although these procedures and criteria were not<br />
described in detail and may ostensibly be exposed to similar biases.<br />
Table 39 Patient criteria of included studies focusing on the use of HBOT in acute myocardial<br />
infarction.<br />
First Author and<br />
Year of Publication<br />
Stavitsky 1998 77<br />
Thurston 1973 78<br />
Patient Criteria<br />
Patients aged between 18 and 80 years admitted to the emergency department showing signs<br />
(ST elevation ≥ 1 mm in two adjacent electrocardiograph leads) or symptoms (chest pain ≥ 20<br />
minutes but ≤ 6 hours in duration unrelieved by sublingual nitroglycerin) suggestive of an acute<br />
myocardial infarction.<br />
Patients under 70 years of age with a strong clinical probability of acute myocardial infarction<br />
occurring within 24 hours of enrolment, and without contraindications to HBO.<br />
Interventions examined<br />
Both studies made use of “conventional therapy” (Table 40) for the comparison group,<br />
but this has changed considerably over the 25 year interval between the identified studies.<br />
The use of more effective therapy over time that increased the probability of better