X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
The energy of the emitted photon will be equal to the difference in energies between the<br />
two orbitals occupied by the electron making the transition. Due to the fact that the energy<br />
difference between two specific orbital shells, in a given element, is always the same (i.e.,<br />
characteristic of a particular element), the photon emitted when an electron moves between<br />
these two levels will always have the same energy. Therefore, by determining the energy<br />
(wavelength) of the X-ray light (photons) emitted by a particular element, it is possible to<br />
determine the identity of that element.<br />
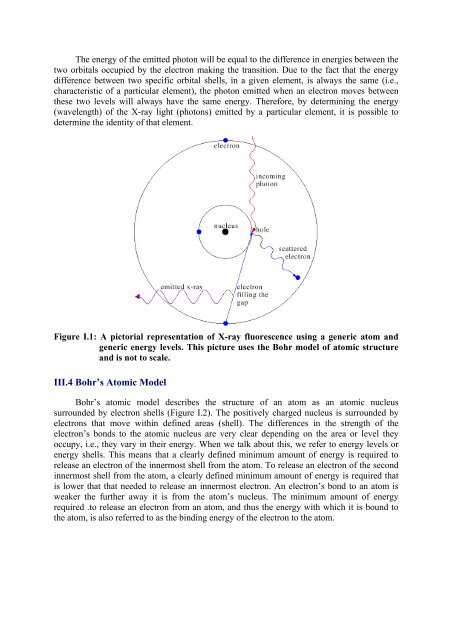
Figure I.1: A pictorial representation of X-ray fluorescence using a generic atom and<br />
generic energy levels. This picture uses the Bohr model of atomic structure<br />
and is not to scale.<br />
III.4 Bohr’s Atomic Model<br />
Bohr’s atomic model describes the structure of an atom as an atomic nucleus<br />
surrounded by electron shells (Figure I.2). The positively charged nucleus is surrounded by<br />
electrons that move within defined areas (shell). The differences in the strength of the<br />
electron’s bonds to the atomic nucleus are very clear depending on the area or level they<br />
occupy, i.e., they vary in their energy. When we talk about this, we refer to energy levels or<br />
energy shells. This means that a clearly defined minimum amount of energy is required to<br />
release an electron of the innermost shell from the atom. To release an electron of the second<br />
innermost shell from the atom, a clearly defined minimum amount of energy is required that<br />
is lower that that needed to release an innermost electron. An electron’s bond to an atom is<br />
weaker the further away it is from the atom’s nucleus. The minimum amount of energy<br />
required .to release an electron from an atom, and thus the energy with which it is bound to<br />
the atom, is also referred to as the binding energy of the electron to the atom.