X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
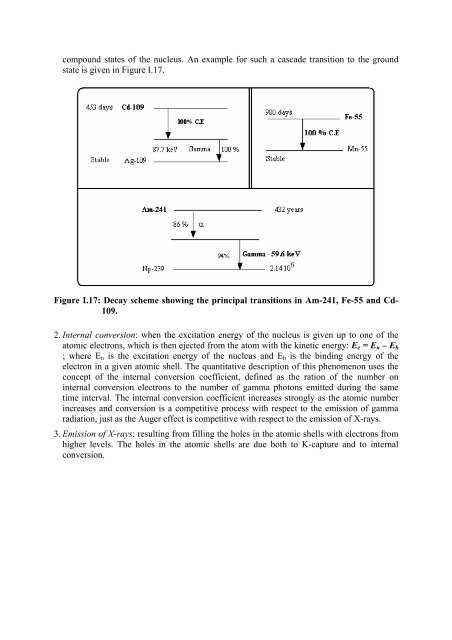
compound states of the nucleus. An example for such a cascade transition to the ground<br />
state is given in Figure I.17.<br />
Figure I.17: Decay scheme showing the principal transitions in Am-241, Fe-55 and Cd-<br />
109.<br />
2. Internal conversion: when the excitation energy of the nucleus is given up to one of the<br />
atomic electrons, which is then ejected from the atom with the kinetic energy: Ee = En – Eb<br />
; where En is the excitation energy of the nucleus and Eb is the binding energy of the<br />
electron in a given atomic shell. The quantitative description of this phenomenon uses the<br />
concept of the internal conversion coefficient, defined as the ration of the number on<br />
internal conversion electrons to the number of gamma photons emitted during the same<br />
time interval. The internal conversion coefficient increases strongly as the atomic number<br />
increases and conversion is a competitive process with respect to the emission of gamma<br />
radiation, just as the Auger effect is competitive with respect to the emission of X-rays.<br />
3. Emission of X-rays: resulting from filling the holes in the atomic shells with electrons from<br />
higher levels. The holes in the atomic shells are due both to K-capture and to internal<br />
conversion.