X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
X-Ray Fluorescence Analytical Techniques - CNSTN : Centre ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
just above the absorption edges of the elements of interest in the sample. Therefore, secondary<br />
target excitation has some obvious advantages over direct tube excitation: its flexibility for<br />
getting an optimized and near monochromatic excitation providing a better selectivity and an<br />
improved sensitivity. However, to compensate for the intensity losses that occur at the<br />
secondary scatterer, a high-powered tube as used in WD spectrometers is required; making<br />
the whole system more sophisticated and expensive compared to direct tube excitation setups.<br />
II.1.3 Radio-Isotopic Excitation<br />
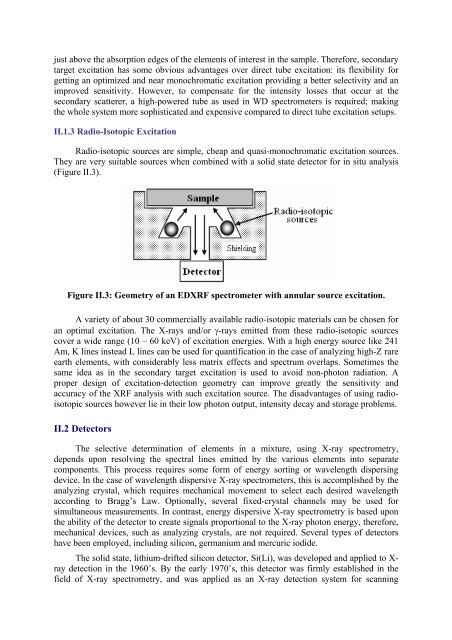
Radio-isotopic sources are simple, cheap and quasi-monochromatic excitation sources.<br />
They are very suitable sources when combined with a solid state detector for in situ analysis<br />
(Figure II.3).<br />
Figure II.3: Geometry of an EDXRF spectrometer with annular source excitation.<br />
A variety of about 30 commercially available radio-isotopic materials can be chosen for<br />
an optimal excitation. The X-rays and/or γ-rays emitted from these radio-isotopic sources<br />
cover a wide range (10 – 60 keV) of excitation energies. With a high energy source like 241<br />
Am, K lines instead L lines can be used for quantification in the case of analyzing high-Z rare<br />
earth elements, with considerably less matrix effects and spectrum overlaps. Sometimes the<br />
same idea as in the secondary target excitation is used to avoid non-photon radiation. A<br />
proper design of excitation-detection geometry can improve greatly the sensitivity and<br />
accuracy of the XRF analysis with such excitation source. The disadvantages of using radioisotopic<br />
sources however lie in their low photon output, intensity decay and storage problems.<br />
II.2 Detectors<br />
The selective determination of elements in a mixture, using X-ray spectrometry,<br />
depends upon resolving the spectral lines emitted by the various elements into separate<br />
components. This process requires some form of energy sorting or wavelength dispersing<br />
device. In the case of wavelength dispersive X-ray spectrometers, this is accomplished by the<br />
analyzing crystal, which requires mechanical movement to select each desired wavelength<br />
according to Bragg’s Law. Optionally, several fixed-crystal channels may be used for<br />
simultaneous measurements. In contrast, energy dispersive X-ray spectrometry is based upon<br />
the ability of the detector to create signals proportional to the X-ray photon energy, therefore,<br />
mechanical devices, such as analyzing crystals, are not required. Several types of detectors<br />
have been employed, including silicon, germanium and mercuric iodide.<br />
The solid state, lithium-drifted silicon detector, Si(Li), was developed and applied to Xray<br />
detection in the 1960’s. By the early 1970’s, this detector was firmly established in the<br />
field of X-ray spectrometry, and was applied as an X-ray detection system for scanning