Micro-gasification: Cooking with gas from biomass - Amper
Micro-gasification: Cooking with gas from biomass - Amper
Micro-gasification: Cooking with gas from biomass - Amper
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Micro</strong>-<strong><strong>gas</strong>ification</strong>: <strong>Cooking</strong> <strong>with</strong> <strong>gas</strong> <strong>from</strong> dry <strong>biomass</strong><br />
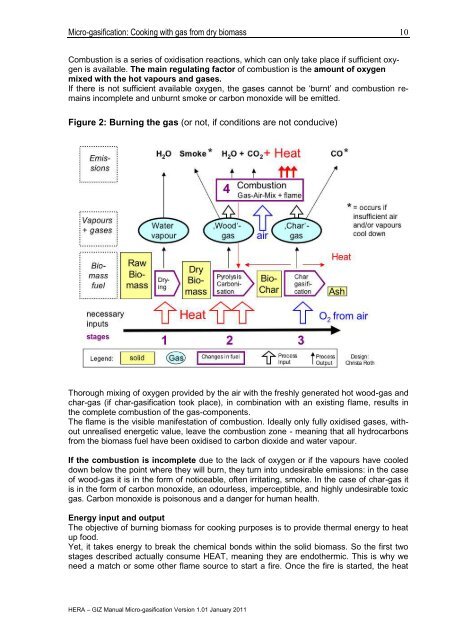
Combustion is a series of oxidisation reactions, which can only take place if sufficient oxygen<br />
is available. The main regulating factor of combustion is the amount of oxygen<br />
mixed <strong>with</strong> the hot vapours and <strong>gas</strong>es.<br />
If there is not sufficient available oxygen, the <strong>gas</strong>es cannot be ‗burnt‘ and combustion remains<br />
incomplete and unburnt smoke or carbon monoxide will be emitted.<br />
Figure 2: Burning the <strong>gas</strong> (or not, if conditions are not conducive)<br />
Thorough mixing of oxygen provided by the air <strong>with</strong> the freshly generated hot wood-<strong>gas</strong> and<br />
char-<strong>gas</strong> (if char-<strong><strong>gas</strong>ification</strong> took place), in combination <strong>with</strong> an existing flame, results in<br />
the complete combustion of the <strong>gas</strong>-components.<br />
The flame is the visible manifestation of combustion. Ideally only fully oxidised <strong>gas</strong>es, <strong>with</strong>out<br />
unrealised energetic value, leave the combustion zone - meaning that all hydrocarbons<br />
<strong>from</strong> the <strong>biomass</strong> fuel have been oxidised to carbon dioxide and water vapour.<br />
If the combustion is incomplete due to the lack of oxygen or if the vapours have cooled<br />
down below the point where they will burn, they turn into undesirable emissions: in the case<br />
of wood-<strong>gas</strong> it is in the form of noticeable, often irritating, smoke. In the case of char-<strong>gas</strong> it<br />
is in the form of carbon monoxide, an odourless, imperceptible, and highly undesirable toxic<br />
<strong>gas</strong>. Carbon monoxide is poisonous and a danger for human health.<br />
Energy input and output<br />
The objective of burning <strong>biomass</strong> for cooking purposes is to provide thermal energy to heat<br />
up food.<br />
Yet, it takes energy to break the chemical bonds <strong>with</strong>in the solid <strong>biomass</strong>. So the first two<br />
stages described actually consume HEAT, meaning they are endothermic. This is why we<br />
need a match or some other flame source to start a fire. Once the fire is started, the heat<br />
HERA – GIZ Manual <strong>Micro</strong>-<strong><strong>gas</strong>ification</strong> Version 1.01 January 2011<br />
10