Micro-gasification: Cooking with gas from biomass - Amper
Micro-gasification: Cooking with gas from biomass - Amper
Micro-gasification: Cooking with gas from biomass - Amper
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Micro</strong>-<strong><strong>gas</strong>ification</strong>: <strong>Cooking</strong> <strong>with</strong> <strong>gas</strong> <strong>from</strong> dry <strong>biomass</strong><br />
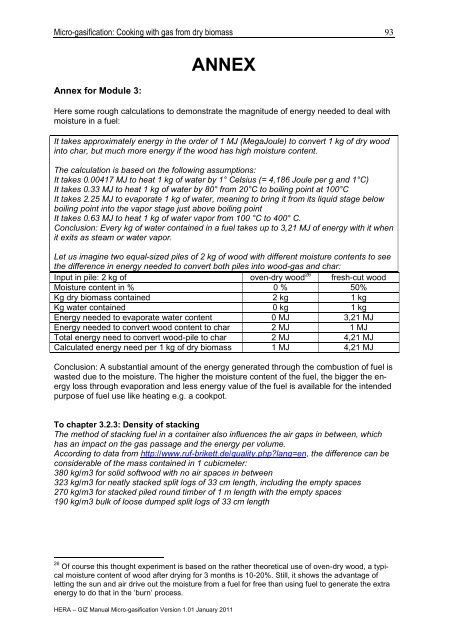
Annex for Module 3:<br />
ANNEX<br />
Here some rough calculations to demonstrate the magnitude of energy needed to deal <strong>with</strong><br />
moisture in a fuel:<br />
It takes approximately energy in the order of 1 MJ (MegaJoule) to convert 1 kg of dry wood<br />
into char, but much more energy if the wood has high moisture content.<br />
The calculation is based on the following assumptions:<br />
It takes 0.00417 MJ to heat 1 kg of water by 1° Celsius (= 4,186 Joule per g and 1°C)<br />
It takes 0.33 MJ to heat 1 kg of water by 80° <strong>from</strong> 20°C to boiling point at 100°C<br />
It takes 2.25 MJ to evaporate 1 kg of water, meaning to bring it <strong>from</strong> its liquid stage below<br />
boiling point into the vapor stage just above boiling point<br />
It takes 0.63 MJ to heat 1 kg of water vapor <strong>from</strong> 100 °C to 400° C.<br />
Conclusion: Every kg of water contained in a fuel takes up to 3,21 MJ of energy <strong>with</strong> it when<br />
it exits as steam or water vapor.<br />
Let us imagine two equal-sized piles of 2 kg of wood <strong>with</strong> different moisture contents to see<br />
the difference in energy needed to convert both piles into wood-<strong>gas</strong> and char:<br />
Input in pile: 2 kg of oven-dry wood 26 fresh-cut wood<br />
Moisture content in % 0 % 50%<br />
Kg dry <strong>biomass</strong> contained 2 kg 1 kg<br />
Kg water contained 0 kg 1 kg<br />
Energy needed to evaporate water content 0 MJ 3,21 MJ<br />
Energy needed to convert wood content to char 2 MJ 1 MJ<br />
Total energy need to convert wood-pile to char 2 MJ 4,21 MJ<br />
Calculated energy need per 1 kg of dry <strong>biomass</strong> 1 MJ 4,21 MJ<br />
Conclusion: A substantial amount of the energy generated through the combustion of fuel is<br />
wasted due to the moisture. The higher the moisture content of the fuel, the bigger the energy<br />
loss through evaporation and less energy value of the fuel is available for the intended<br />
purpose of fuel use like heating e.g. a cookpot.<br />
To chapter 3.2.3: Density of stacking<br />
The method of stacking fuel in a container also influences the air gaps in between, which<br />
has an impact on the <strong>gas</strong> passage and the energy per volume.<br />
According to data <strong>from</strong> http://www.ruf-brikett.de/quality.php?lang=en, the difference can be<br />
considerable of the mass contained in 1 cubicmeter:<br />
380 kg/m3 for solid softwood <strong>with</strong> no air spaces in between<br />
323 kg/m3 for neatly stacked split logs of 33 cm length, including the empty spaces<br />
270 kg/m3 for stacked piled round timber of 1 m length <strong>with</strong> the empty spaces<br />
190 kg/m3 bulk of loose dumped split logs of 33 cm length<br />
26 Of course this thought experiment is based on the rather theoretical use of oven-dry wood, a typical<br />
moisture content of wood after drying for 3 months is 10-20%. Still, it shows the advantage of<br />
letting the sun and air drive out the moisture <strong>from</strong> a fuel for free than using fuel to generate the extra<br />
energy to do that in the ‗burn‘ process.<br />
HERA – GIZ Manual <strong>Micro</strong>-<strong><strong>gas</strong>ification</strong> Version 1.01 January 2011<br />
93