Research Report 2010 2011 - Helmholtz-Zentrum für ...
Research Report 2010 2011 - Helmholtz-Zentrum für ...
Research Report 2010 2011 - Helmholtz-Zentrum für ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SCIENTIFIC REPORTS | Infection and Immunity | Strategies for Prevention and Therapy 103<br />
04.2 Interaction between Innate and Adaptive Immunity<br />
PROJECT LEADER | Prof. Dr. Ulrich Kalinke | <strong>Research</strong> Group Experimental Infection <strong>Research</strong> | Twincore |<br />
ulrich.kalinke@twincore.de<br />
PROJECT MEMBERS | Dr. Claudia Detje | Dr. Theresa Frenz | Elena Grabski | Sabrina Heindorf | Julia Heinrich |<br />
Christian Mers | Claudia Soldner<br />
Following viral infection, type I interferon responses that<br />
secure the initial survival of the host are usually induced<br />
within hours. It is approximately a week later that adaptive<br />
immunity is activated to an extent that it is able to eradicate<br />
the infecting pathogen. In earlier studies we showed that<br />
upon viral infection a small number of highly specialised<br />
immune cells, also addressed as plasmacytoid dendritic cells<br />
(pDC), produce large quantities of protective type I interferon.<br />
Practically all viruses examined more closely developed<br />
countermeasures that inhibit the induction or the function<br />
of such type I interferon responses. We are investigating how<br />
different viruses induce type I interferon responses, which<br />
strategies they have developed to undermine these responses<br />
and how interferons secure the survival of the host.<br />
Mechanisms of type I interferon induction and its protective<br />
function The molecular mechanism of type I interferon<br />
induction may vary depending on the inducing agent and the<br />
analysed tissue of cell type. We study in the mouse model<br />
how the entry of the vesicular stomatitis virus (VSV) into the<br />
central nervous system via the olfactory system is inhibited<br />
interferon-dependently (see also Figure 1). Furthermore, we<br />
analyse the influence type I interferon responses have on<br />
immune cell functions. In this context, we have found that a<br />
vaccinia virus-derived attenuated pathogen variant, modified<br />
vaccinia virus (MVA), which triggers strong type I interferon<br />
responses, also induces a type I interferon-dependent expansion<br />
of virus-specific T cells. In this context type I interferon<br />
stimulation of both the antigen-presenting dendritic cells as<br />
well as T cells plays a central role. Furthermore, we investigate<br />
the influence type I interferon has on the induction of<br />
long-lasting antibody responses. This work plays an important<br />
role for the development of new vaccination strategies.<br />
Virus-mediated activation of human pDC Some patients<br />
with chronic hepatitis C virus (HCV) infection can be successfully<br />
treated with an interferon-alpha/ribavirin combination<br />
therapy. However, so far little is known about whether<br />
the body’s own interferon system is sufficiently activated<br />
in HCV infection. We investigate which HCV and/or host<br />
encoded factors influence the strength and composition of<br />
HCV-induced cytokine response of human pDC. This work is<br />
carried out in vitro with primary human pDC isolated from<br />
blood samples from healthy donors. In the future also experiments<br />
with pDC from chronically HCV infected patients will<br />
be carried out.<br />
Perspective An improved understanding of the multiple<br />
functions of type I interferon can help to optimize type I<br />
interferon-based therapies of tumors, autoimmune diseases<br />
and viral infections. Furthermore deeper knowledge of viral<br />
mechanisms of immune stimulation and evasion will provide<br />
new approaches for vaccine development.<br />
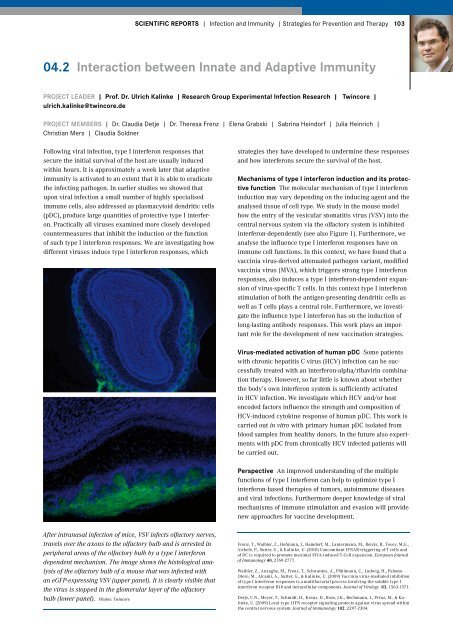
After intranasal infection of mice, VSV infects olfactory nerves,<br />
travels over the axons to the olfactory bulb and is arrested in<br />
peripheral areas of the olfactory bulb by a type I interferon<br />
dependent mechanism. The image shows the histological analysis<br />
of the olfactory bulb of a mouse that was infected with<br />
an eGFP-expressing VSV (upper panel). It is clearly visible that<br />
the virus is stopped in the glomerular layer of the olfactory<br />
bulb (lower panel). Photos: Twincore<br />
Frenz, T., Waibler, Z., Hofmann, J., Hamdorf, M., Lantermann, M., Reizis, B., Tovey, M,G.,<br />
Aichele, P., Sutter, G., & Kalinke, U. (<strong>2010</strong>) Concomitant IFNAR-triggering of T cells and<br />
of DC is required to promote maximal MVA-induced T-Cell expansion. European Journal<br />
of Immunology 40, 2769-2777.<br />
Waibler, Z., Anzaghe, M., Frenz, T., Schwantes, A., Pöhlmann, C., Ludwig, H., Palomo-<br />
Otero, M., Alcamí, A., Sutter, G., & Kalinke, U. (2009) Vaccinia virus-mediated inhibition<br />
of type I interferon responses is a multifactorial process involving the soluble type I<br />
interferon receptor B18 and intracellular components. Journal of Virology 83, 1563-1571.<br />
Detje, C.N., Meyer, T., Schmidt, H., Kreuz, D., Rose, J.K., Bechmann, I., Prinz, M., & Kalinke,<br />
U. (2009) Local type I IFN receptor signaling protects against virus spread within<br />
the central nervous system. Journal of Immunology 182, 2297-2304.