Research Report 2010 2011 - Helmholtz-Zentrum für ...
Research Report 2010 2011 - Helmholtz-Zentrum für ...
Research Report 2010 2011 - Helmholtz-Zentrum für ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
104 SCIENTIFIC REPORTS | Infection and Immunity | Strategies for Prevention and Therapy<br />
04.3 Antigen Delivery Systems and Vaccines<br />
PROJECT LEADER | Prof. Carlos A. Guzmán, M.D., Ph.D. | Department of Vaccinology and Applied Microbiology |<br />
cag@helmholtz-hzi.de<br />
PROJECT MEMBERS | Dr. Pablo D. Becker | Dr. Jennifer Debarry | Dr. Thomas Ebensen | Dr. Miriam Nörder |<br />
Dr. Peggy Riese | Rimma Libanova | Kirsten Scholz | Dr. Kai Schulze | Sebastian Weissmann | Dr. Beata Zygmunt<br />
The main aim of this project is the development of tools and<br />
strategies to optimize the delivery of vaccine antigens, particularly<br />
by the mucosal route, and their subsequent exploitation<br />
for the generation of vaccines against specific diseases.<br />
Improving antigen design Despite the strong immune<br />
responses elicited after natural infection with Trypanosoma<br />
cruzi or vaccination against it, parasite survival in the host<br />
suggests that these responses are insufficient or inherently<br />
inadequate. T. cruzi contains a major cystein proteinase,<br />
cruzipain, which is an attractive candidate vaccine antigen.<br />
We produced full-length recombinant cruzipain (rCz), as<br />
well as truncated forms encompassing its N- and C-terminal<br />
domains. Groups of mice were then immunized with the<br />
recombinant proteins combined with CpG-ODN and Czspecific<br />
antibody responses were measured. The highest<br />
IgG titers were observed in mice vaccinated with either the<br />
C-terminal domain or the full-length protein. In contrast, low<br />
antibody titers were stimulated in mice immunized with the<br />
N-terminal domain, thereby suggesting a parasite immune<br />
escape mechanism by avoiding antibody production against<br />
the catalytic N-terminal domain. Similarly, cellular responses<br />
were poorly restimulated with the N-terminal domain in<br />
mice immunized with full-length rCz, whereas most of the<br />
response was evoked by the C-terminal domain. However, the<br />
cellular responses were much stronger when the N-terminal<br />
domain was used for immunization, suggesting that cellular<br />
responses can be down-regulated by full-length Cz to avoid<br />
recognition of the N-terminal domain. Masking of the essential<br />
domain can be reverted by using the N-terminal domain as<br />
a tailored immunogen to redirect host responses to provide<br />
enhanced protection. In fact, mice immunized with the N-<br />
terminal domain were able to better control infection, resulting<br />
in significant lower parasite loads throughout the acute<br />
phase and limited tissue injury during the chronic phase.<br />
Optimizing vaccination strategies Mucosal vaccination<br />
is an attractive strategy for antigen administration, since<br />
this approach is not associated with pain or stress, has an<br />
extremely easy and cost-efficient administration logistics<br />
and does not require highly trained personnel. However,<br />
although many features of the mucosal immune system<br />
have been dissected, there is an incomplete knowledge on<br />
the effector mechanisms triggered by mucosal vaccination.<br />
In this context, the stimulation of specific T helper (Th)<br />
subsets is critical to promote efficient immunity without<br />
side effects. Thus, we investigated which Th subsets are<br />
preferentially stimulated following intranasal immunization.<br />
In comparison to parenteral immunization we detected<br />
an up-regulation of CCR-6 on CD4+ cells which correlated<br />
with an enhanced production of IL-17. This preferential<br />
induction of Th17 immune response is independent from<br />
the kind of adjuvant used.<br />
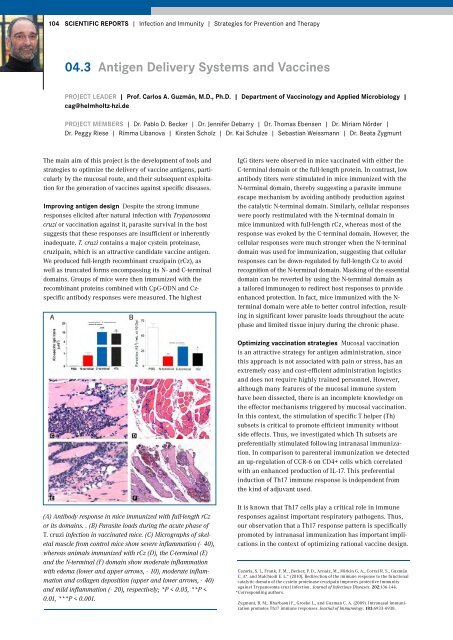
(A) Antibody response in mice immunized with full-length rCz<br />
or its domains. . (B) Parasite loads during the acute phase of<br />
T. cruzi infection in vaccinated mice. (C) Micrographs of skeletal<br />
muscle from control mice show severe inflammation (×40),<br />
whereas animals immunized with rCz (D), the C-terminal (E)<br />
and the N-terminal (F) domain show moderate inflammation<br />
with edema (lower and upper arrows, ×10), moderate inflammation<br />
and collagen deposition (upper and lower arrows, ×40)<br />
and mild inflammation (×20), respectively; *P < 0.05, **P <<br />
0.01, ***P < 0.001.<br />
It is known that Th17 cells play a critical role in immune<br />
responses against important respiratory pathogens. Thus,<br />
our observation that a Th17 response pattern is specifically<br />
promoted by intranasal immunization has important implications<br />
in the context of optimizing rational vaccine design.<br />
Cazorla, S. I., Frank, F. M. , Becker, P. D., Arnaiz, M., Mirkin G. A., Corral R. S., Guzmán<br />
C. A*. and Malchiodi E. L.* (<strong>2010</strong>). Redirection of the immune response to the functional<br />
catalytic domain of the cystein proteinase cruzipain improves protective immunity<br />
against Trypanosoma cruzi infection. Journal of Infectious Diseases. 202:136-144.<br />
*Corresponding authors.<br />
Zygmunt, B. M., Rharbaoui F., Groebe L., and Guzman C. A. (2009). Intranasal immunization<br />
promotes Th17 immune responses. Journal of Immunology. 183:6933-6938.