2010 Progress Report - International Joint Commission
2010 Progress Report - International Joint Commission
2010 Progress Report - International Joint Commission
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Reduction in surface water calcium concentrations<br />
has several ecological implications, although many<br />
impacts are not well quantifi ed yet. Populations of<br />
calcium-rich zooplankton (e.g. Daphnia species)<br />
occur in many Canadian Shield lakes. In the Muskoka<br />
region of Ontario, the mean calcium concentration<br />
of 36 lakes declined 13% between 1985 and 2005.<br />
Contemporaneous evaluation of the relative abundance<br />
of calcium-rich daphniids in 43 Muskoka lakes showed<br />
that they have declined in 60% of the lakes having a<br />
present-day calcium concentration less than 1.5 mg L -1<br />
(the level at which reproduction is delayed), and in<br />
Recovery of Acidified Lakes<br />
and Streams<br />
Acid rain, resulting from SO 2<br />
and NO x<br />
emissions, is one of many large-scale<br />
anthropogenic effects that negatively<br />
affect the health of lakes and streams<br />
in the United States and Canada.<br />
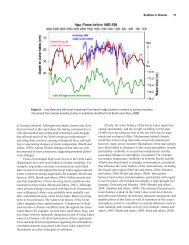
Surface water chemistry provides direct indicators of<br />
the potential effects of acidic deposition on the overall<br />
health of aquatic ecosystems.<br />
UNITED STATES<br />
Three indicators of acidity in surface waters provide<br />
information regarding both sensitivity to surface water<br />
acidifi cation and the level of acidifi cation that has<br />
occurred today and in the past. These indicators are<br />
sulphate ions (SO 4<br />
2-<br />
), nitrate ions and acid-neutralizing<br />
capacity (ANC). Sulphate and nitrate are negatively<br />
charged ions with the potential to acidify drainage<br />
waters and leach acidic aluminum cations from<br />
watershed soils. Aluminium cations are known to be<br />
toxic to aquatic life. Assessments of acidic deposition<br />
effects dating from the 1970s to the present have<br />
shown sulphate to be the primary negatively charged<br />
ion in most acid-sensitive waters.<br />
Long-term monitoring networks, such as the U.S.<br />
EPA’s Long-Term Monitoring (LTM) program, provide<br />
information on the chemistry of lakes and streams,<br />
which allow us to look at how water bodies respond<br />
to changing emissions. The LTM program monitors a<br />
total of 170 lakes and streams, representing the major<br />
acid-sensitive regions of the northern and eastern<br />
67% of the lakes having present-day calcium between<br />
1.5 and 2.0 mg L -1 . Because calcium-rich daphniids<br />
are often the most abundant zooplankton in the lake<br />
environment, the population decline may affect the<br />
entire food web. Hence, declining calcium levels are<br />
expected to affect fi sh and other aquatic species as<br />
well, and the effects may even extend outside of the<br />
aquatic environment to the birds and animals that<br />
depend on the lakes for food. Studies indicate that<br />
even after recovery of lake pH, continued low levels of<br />
calcium could prevent full population recovery of the<br />
daphniids to pre-impact levels.<br />
• Sulphate ion concentrations in surface waters<br />
provide important information on the extent<br />
of base cation (i.e. calcium, magnesium,<br />
potassium and sodium) leaching in soils and<br />
offer insight on how sulphate concentrations<br />
relate to the levels of ambient atmospheric<br />
sulphur and atmospheric deposition.<br />
• Nitrogen is an important nutrient for plant<br />
growth and, therefore, most nitrogen inputs<br />
by deposition are quickly incorporated<br />
into biomass during the growing season as<br />
organic nitrogen, with little leaching of nitrate<br />
into surface waters during the growing season.<br />
As atmospheric nitrogen deposition increases,<br />
there is greater potential for increased leaching<br />
of nitrate into surface waters.<br />
• ANC is a measure of the acid-buffering<br />
capacity of water and an important<br />
indicator of the sensitivity and the degree of<br />
surface water acidifi cation or recovery that<br />
occurs over time. Acidifi cation results in a<br />
diminishing ability of water in the lake or<br />
stream to neutralize strong acids that enter<br />
aquatic ecosystems.<br />
United States (New England, Adirondack Mountains,<br />
northern Appalachian Plateau, and Ridge/Blue Ridge<br />
provinces of Virginia).<br />
Scientific and Technical Cooperation and Research<br />
63