Fundamental Organic Chemistry
Fundamental Organic Chemistry
Fundamental Organic Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
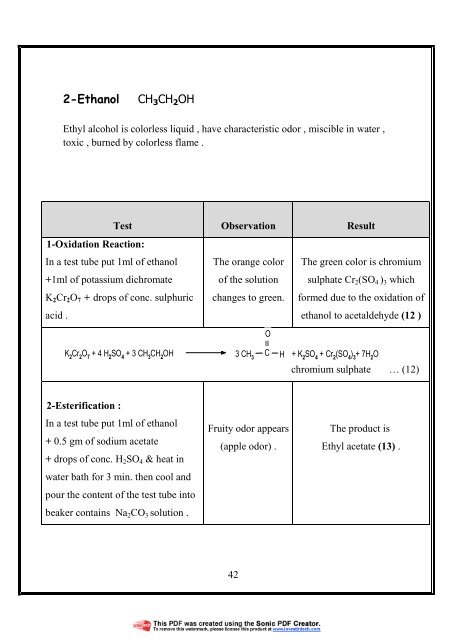
2-Ethanol<br />
CH 3 CH 2 OH<br />
Ethyl alcohol is colorless liquid , have characteristic odor , miscible in water ,<br />
toxic , burned by colorless flame .<br />
Test<br />
1-Oxidation Reaction:<br />
In a test tube put 1ml of ethanol<br />
+1ml of potassium dichromate<br />
K 2 Cr 2 O 7 + drops of conc. sulphuric<br />
acid .<br />
Observation<br />
The orange color<br />
of the solution<br />
changes to green.<br />
O<br />
Result<br />
The green color is chromium<br />
sulphate Cr 2 (SO 4 ) 3 which<br />
formed due to the oxidation of<br />
ethanol to acetaldehyde (12 )<br />
K 2<br />
Cr 2<br />
O 7<br />
+ 4 H 2<br />
SO 4<br />
+ 3 CH 3<br />
CH 2<br />
OH 3 CH 3<br />
C H<br />
+ K 2<br />
SO 4<br />
+ Cr 2<br />
(SO 4<br />
) 3<br />
+ 7H 2<br />
O<br />
chromium sulphate … (12)<br />
2-Esterification :<br />
In a test tube put 1ml of ethanol<br />
+ 0.5 gm of sodium acetate<br />
+ drops of conc. H 2 SO 4 & heat in<br />
water bath for 3 min. then cool and<br />
pour the content of the test tube into<br />
beaker contains Na 2 CO 3 solution .<br />
Fruity odor appears<br />
(apple odor) .<br />
The product is<br />
Ethyl acetate (13) .<br />
42