Fundamental Organic Chemistry
Fundamental Organic Chemistry
Fundamental Organic Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
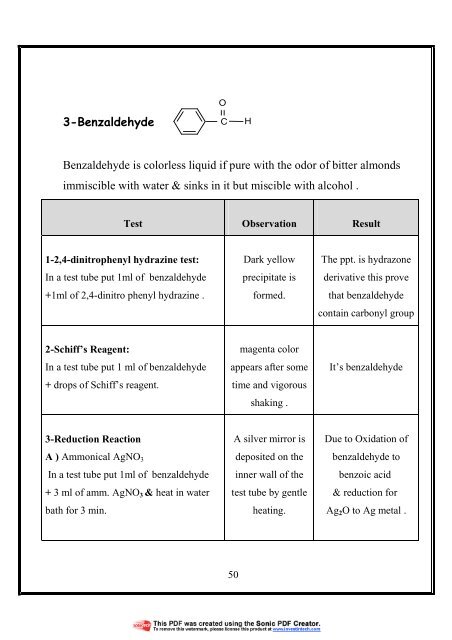
3-Benzaldehyde<br />
O<br />
C<br />
H<br />
Benzaldehyde is colorless liquid if pure with the odor of bitter almonds<br />
immiscible with water & sinks in it but miscible with alcohol .<br />
Test<br />
Observation<br />
Result<br />
1-2,4-dinitrophenyl hydrazine test:<br />
In a test tube put 1ml of benzaldehyde<br />
+1ml of 2,4-dinitro phenyl hydrazine .<br />
Dark yellow<br />
precipitate is<br />
formed.<br />
The ppt. is hydrazone<br />
derivative this prove<br />
that benzaldehyde<br />
contain carbonyl group<br />
2-Schiff’s Reagent:<br />
In a test tube put 1 ml of benzaldehyde<br />
+ drops of Schiff’s reagent.<br />
magenta color<br />
appears after some<br />
time and vigorous<br />
shaking .<br />
It’s benzaldehyde<br />
3-Reduction Reaction<br />
A ) Ammonical AgNO 3<br />
In a test tube put 1ml of benzaldehyde<br />
+ 3 ml of amm. AgNO 3 & heat in water<br />
bath for 3 min.<br />
A silver mirror is<br />
deposited on the<br />
inner wall of the<br />
test tube by gentle<br />
heating.<br />
Due to Oxidation of<br />
benzaldehyde to<br />
benzoic acid<br />
& reduction for<br />
Ag 2 O to Ag metal .<br />
50