Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
Electrons and Quantum Mechanics - Oakland Schools
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
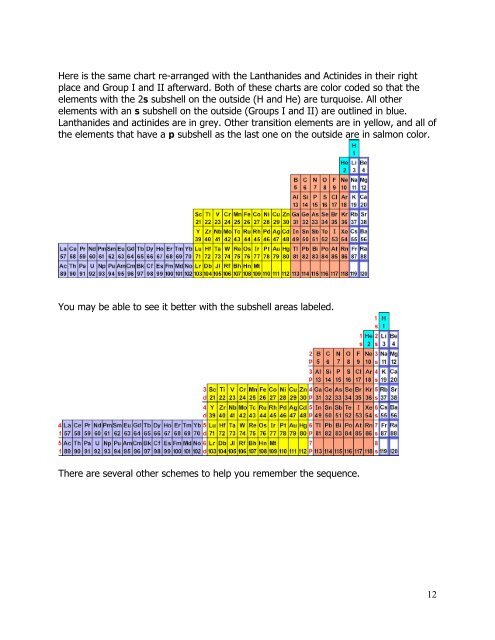
Here is the same chart re-arranged with the Lanthanides <strong>and</strong> Actinides in their right<br />
place <strong>and</strong> Group I <strong>and</strong> II afterward. Both of these charts are color coded so that the<br />
elements with the 2s subshell on the outside (H <strong>and</strong> He) are turquoise. All other<br />
elements with an s subshell on the outside (Groups I <strong>and</strong> II) are outlined in blue.<br />
Lanthanides <strong>and</strong> actinides are in grey. Other transition elements are in yellow, <strong>and</strong> all of<br />
the elements that have a p subshell as the last one on the outside are in salmon color.<br />
You may be able to see it better with the subshell areas labeled.<br />
There are several other schemes to help you remember the sequence.<br />
12